Organic Chemistry II |
 |
|
Professor Carl C. Wamser |
||
Chem 335 - Winter Term |
Quiz 13 |
![]()
Organic Chemistry II |
 |
|
Professor Carl C. Wamser |
||
Chem 335 - Winter Term |
Quiz 13 |
![]()
1. (2 points) How many different peaks would be expected in the C-13 NMR spectrum (decoupled) for 1,5-dimethylnaphthalene?
What is its molecular formula and IHD?
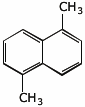 6 different C-13 peaks
6 different C-13 peaks2. (2 points) Explain how you could distinguish between the ortho and para isomers below.
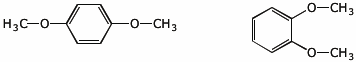
using H-NMR:
para - two singlets, one for the CH3 and one for the four equivalent aryl Hs
ortho - one singlet for the CH3 groups and two doublets for the aryl Hs
using C-13 NMR:
para - total of 3 peaks
ortho - total of 4 peaks
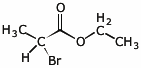
3. (6 points) Identify the unknown compound that gives the following spectral data. Indicate what information you gain from each bit of data.
molecular formula - C5H9BrO2
IHD = 1
mass spec - m/z = 180, 182 (approximately equal intensities)
molecule contains one Br
infrared - strong peak at 1740 cm-1
molecule contains a C=O
H-NMR -
1.3 ppm, 3H, triplet
1.8 ppm, 3H, doublet
4.2 ppm, 2H, quartet
4.4 ppm, 1H, quartet