Organic Chemistry II |
 |
|
Professor Carl C. Wamser |
||
Chem 335 - Winter Term |
Final Exam Answer Key |
![]()
Organic Chemistry II |
 |
|
Professor Carl C. Wamser |
||
Chem 335 - Winter Term |
Final Exam Answer Key |
![]()
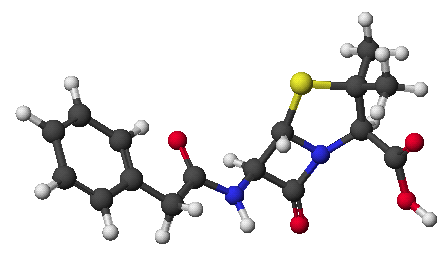
1. (25 points) Write complete names for each of the following.
a) 
b) 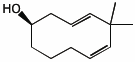
c) 
d) 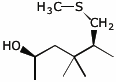
e) 
2. (15 points) Write complete structures for the following.
a) a 3° benzylic alcohol
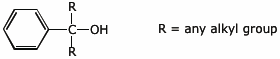
b) the HOMO of (E,E)-2,4-hexadiene
![]()
c) a good dienophile of formula C4H2N2

d) lithium diisobutylcuprate
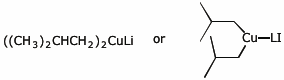
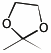
e) the acetal made from acetone (propanone) and ethylene glycol (1,2-ethanediol)
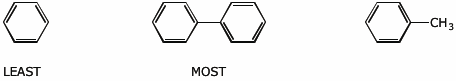
3. (15 pts) Arrange the following in order with respect to the property indicated. Write MOST and LEAST under the compounds with the highest and lowest values, respectively.
a) reactivity in nitration
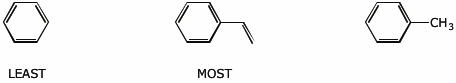
b) reactivity towards Br2
c) reactivity in SN1 solvolysis
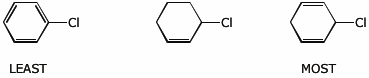
d) acidity
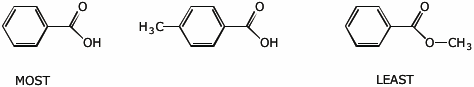
e) relative stability
![]()
4. (15 pts) Complete each of the following reactions by adding the missing part: either the starting materials, the necessary reagents and conditions, or the expected major product.
a) 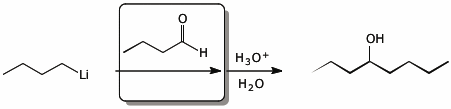
b) 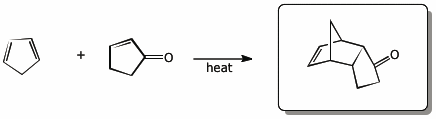
c) 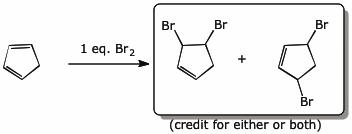
d) 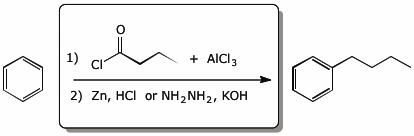
e) 
5. (15 pts) Complete each of the following reactions by adding the missing part: either the starting materials, the necessary reagents and conditions, or the expected major product.
a) 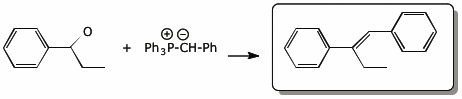
b) 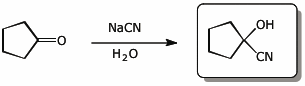
c) 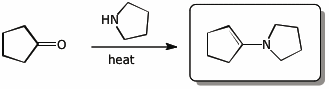
d) 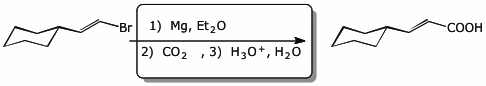
e)
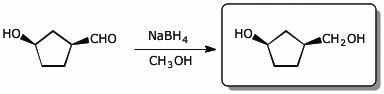
6. (15 points) All the compounds below undergo hydrolysis in dilute aqueous acid. Show the products of hydrolysis in each case.
a) 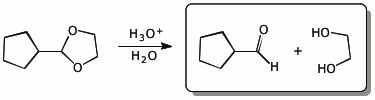
b) 
c) 
d) 
e) 
7. (15 points) Write a complete mechanism for the hydrolysis reaction shown below. Show all steps in the mechanism and all resonance forms for any intermediates. Electron-pushing arrows are optional.
![]()
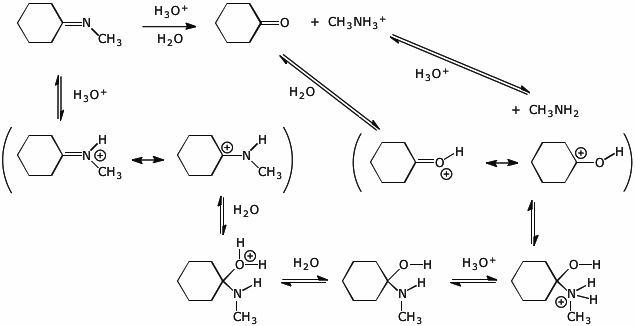
8. (15 points) Write a complete mechanism for the Fischer esterification shown below. Show all steps in the mechanism and all resonance forms for any intermediates. Electron-pushing arrows are optional.
![]()

9. (15 points) Write a complete mechanism for the dehydration reaction shown below. Show all steps in the mechanism and all resonance forms for any intermediates. Electron-pushing arrows are optional.
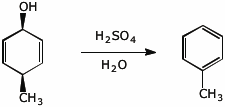

10. (20 points) Show how you would prepare the following compound by two different routes. All carbons in your final product must originate from benzene and/or any alcohol with four or fewer carbons.

a) using a Grignard addition to an aldehyde
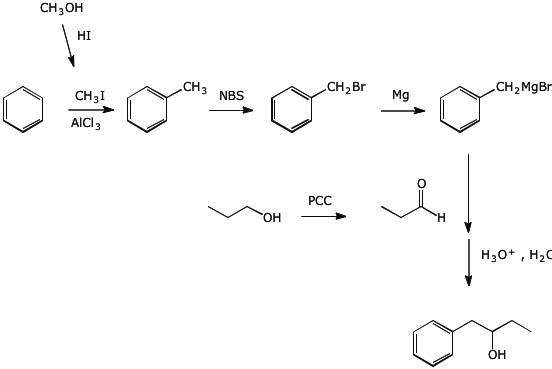
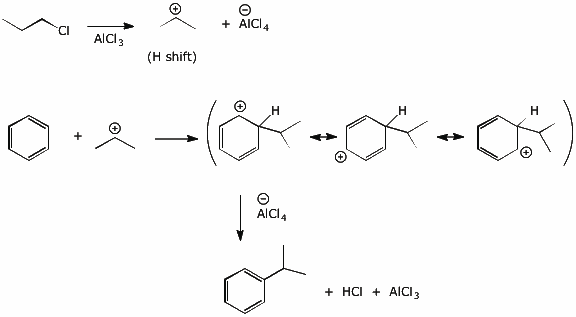
b) using a Grignard addition to an epoxide

11. (15 points) Stu Dent attempted to prepare propylbenzene by a Friedel-Crafts alkylation of benzene using 1-chloropropane and AlCl3. The proton NMR spectrum of the product is shown below.
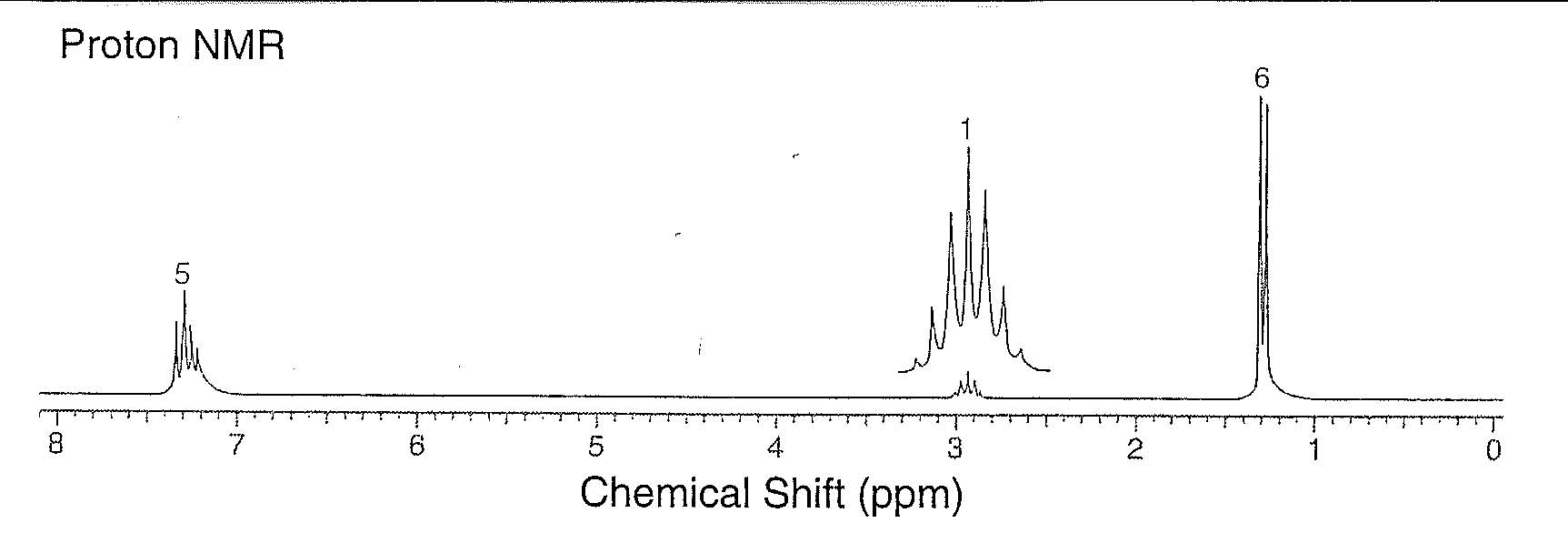
Identify the structure of the product and correlate each absorption in the spectrum with the appropriate Hs in the molecule.

Write a complete mechanism for the reaction to form this product. Show all steps in the mechanism and all resonance forms for any intermediates. Electron-pushing arrows are optional.
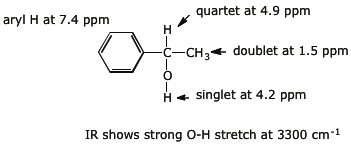
12. (10 points) Identify the unknown compound based on the information provided. Identify the structural features in your molecule that correlate with each of the spectral features.
Compound X has the molecular formula C8H10O .

13. (10 points) Identify the unknown compound based on the information provided. Identify the structural features in your molecule that correlate with each of the spectral features.
Compound Y has the molecular formula C7H12O4 .
IR (cm-1) : 3050 (strong, broad), 1750 (strong)
C-13 NMR (ppm) : 180, 65, 55, 40, 35, 25
H- NMR:
11.3 ppm, 1H (singlet)
3.9 ppm, 4H (singlet)
2.5 ppm, 2H (triplet)
2.1 ppm, 2H (triplet)
2.0 ppm, 3H (singlet)
