Organic Chemistry II |
 |
|
Professor Carl C. Wamser |
||
Chem 335 - Winter Term |
Exam 2 Answer Key |
![]()
Organic Chemistry II |
 |
|
Professor Carl C. Wamser |
||
Chem 335 - Winter Term |
Exam 2 Answer Key |
![]()
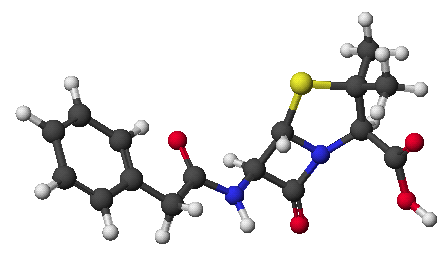
1. (15 points) Write complete names for each of the following
a) 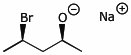
b) ![]()
c) ![]()
d) ![]()
e) 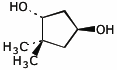
2. (20 pts) Give brief but complete answers for each of the following.
a) Explain how you could use C-13 NMR to distinguish the ortho, meta, and para isomers of the dichlorobenzenes.
ortho has 3 peaks, meta has 4 peaks, para has 2 peaks
b) The mass spectrum of benzyl alcohol shows peaks at m/z = 108, 91, and 77.
Write structures for each of these.
c) Use electron-pushing arrows to illustrate the Simmons-Smith reaction of
cyclopentene with ICH2ZnI.
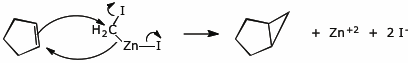
d) Show the ring-closing metathesis reaction of 1,6-heptadiene.
![]()
3. (10 pts) Write a complete mechanism for the reaction shown below, including all steps and all resonance forms for any intermediates. Electron-pushing arrows are optional.

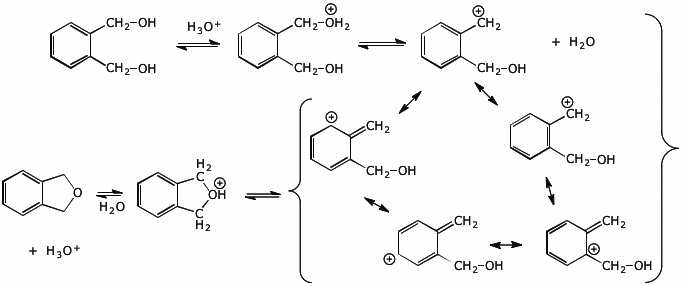
4. (15 pts) Complete each of the following reactions by adding the expected major product.
a) 
b) 
c) 
d) 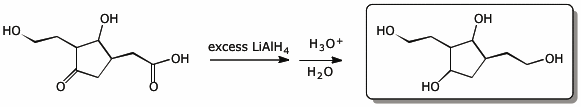
e)
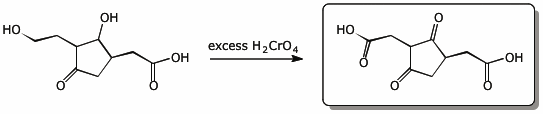
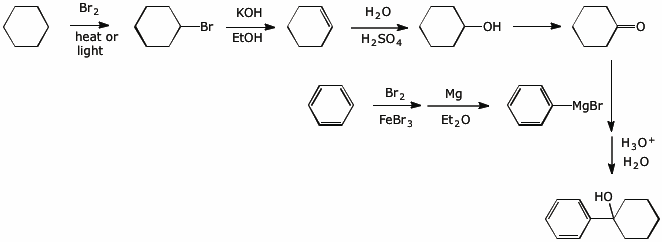
5. (20 points) Write synthetic sequences that show how you would prepare the following compounds.
a) starting from benzene and cyclohexane

b) starting from any alcohols with three or fewer carbons


6. (10 points) Identify the unknown compound that shows the following spectral data.
Correlate each of the spectral features below with a structural feature in your final compound.
Molecular formula - C8 H10 O
The IR spectrum shows a strong broad peak at 3300 cm-1.
The C-13 NMR spectrum shows six peaks at 15, 30, 115, 120, 125, 150 ppm.

IR shows the O-H
C-13 shows CH3 at 15, CH2 at 30, 4 different aryl between 115 and 150
H-1 NMR shows CH3 at 1.2, CH2 at 2.6, aryl H at 6.7 and 7.1
7. (10 points) Identify the unknown compound that shows the following spectral data.
Correlate each of the spectral features below with a structural feature in your final compound.
Molecular formula - C8 H10 O
The IR spectrum shows that there are no O-H or C=O groups present.
The C-13 NMR spectrum shows six peaks at 20, 55, 114, 129, 130, 154 ppm.
Proton NMR
a - 2.3 ppm, 3H, singlet
b - 3.8 ppm, 3H, singlet
c - 6.9 ppm, 2H, doublet
d - 7.2 ppm, 2H, doublet
C-13 shows CH3 at 20, CH3O at 55, 4 different aryl between 114 and 154