Organic Chemistry II |
 |
|
Professor Carl C. Wamser |
||
Chem 335 - Winter Term |
Exam 1 Answer Key |
![]()
Organic Chemistry II |
 |
|
Professor Carl C. Wamser |
||
Chem 335 - Winter Term |
Exam 1 Answer Key |
![]()
1. (14 points) Write complete structures for the following.
a) (4 points) the 1,2- and 1,4- addition products of Br2 to 2,4-hexadiene (ignore stereochemistry)
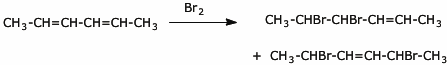
b) (4 points) both HOMOs of benzene

c) (6 points) aromatic compounds that have the following molecular formulae:
C4H5N
![]()
C5H5N
![]()
C6H7N
![]() or any methylpyridine (ethylpyrrole is C6H9N)
or any methylpyridine (ethylpyrrole is C6H9N)
2. (16 points) Write complete structures for the following.
a) (4 points) both enantiomers of 1,3-dichloro-1,2-propadiene

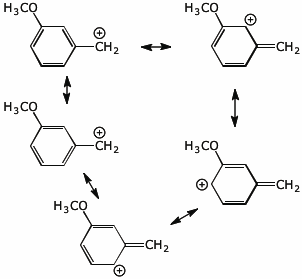
b) (6 points) all the resonance forms for m-methoxybenzyl cation
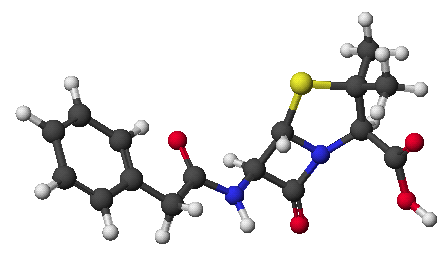
c) (6 points) The nucleic acid adenine includes the heterocyclic structure shown below.
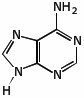
For each of the five nitrogens, identify the expected hybridization of N, and indicate in which orbital the lone pair would be expected.

3. (15 points) Arrange the following in order with respect to the property indicated. Write “MOST” under the compound with the highest value and “LEAST” under the compound with the lowest value.
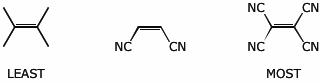
a) reactivity as a dienophile in a Diels-Alder reaction
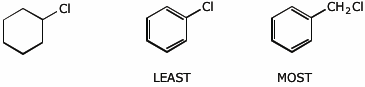
b) reactivity in nucleophilic substitution
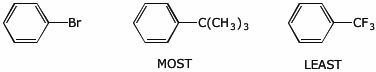
c) reactivity in nucleophilic substitution

d) reactivity in nitration
e) relative stability

4. (15 pts) Complete each of the following reactions by adding the expected major product.
a) 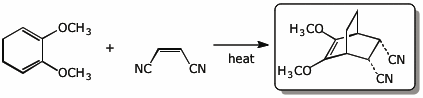
b) 
c) 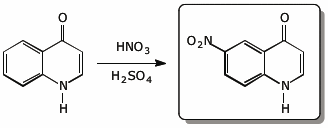
d) 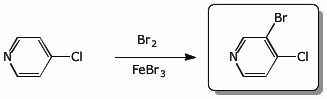
e) 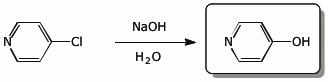
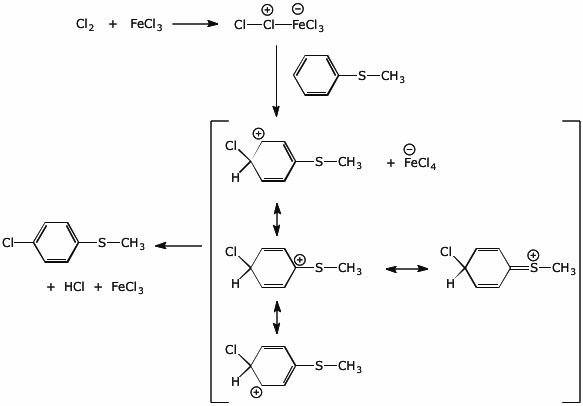
5. (15 points) Predict the properties of a CH3S- substituent in electrophilic aromatic substitution.
Is it activating or deactivating? ACTIVATING
Is it o,p- or m-directing? o,p-directing
Write a complete mechanism for the chlorination of the compound below to illustrate your prediction.
![]()
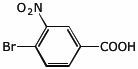
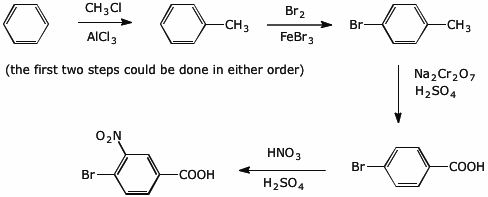
6. (10 points) Show a reaction sequence that could be used to prepare the following compound starting from benzene plus any other needed compounds or reagents.
7. (15 points) Chloroprene, shown below, undergoes 1,4-polymerization to form the common polymer Neoprene. Illustrate the repeat unit of this polymer. If there are stereochemical possibilities, show all isomers.
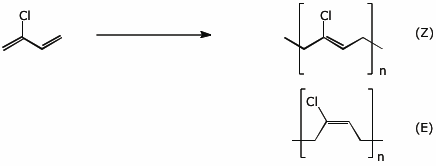
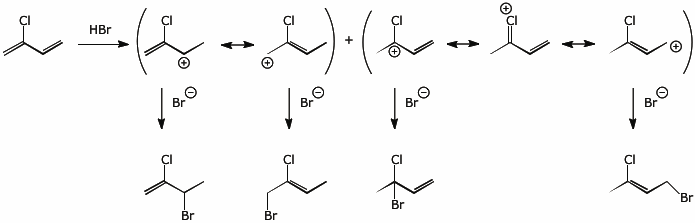
Illustrate both the 1,2- and 1,4- addition of HBr to chloroprene, showing the resonance forms for the expected intermediates.