Organic Chemistry II |
 |
|
Professor Carl C. Wamser |
||
Chem 335 - Winter Term |
Quiz 10 |
![]()
Organic Chemistry II |
 |
|
Professor Carl C. Wamser |
||
Chem 335 - Winter Term |
Quiz 10 |
![]()
1. (2 points) Write the expected major product for the following reactions:
a) 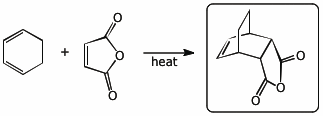
b) 
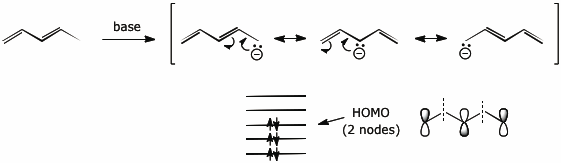
2. (4 points) Removal of a proton from the terminal methyl group of 1,3-pentadiene leads to a carbanion with three resonance forms.
Write all three resonance forms including electron-pushing arrows to illustrate how they are interconverted.
Write the energy-level diagram for this pi system, including the electrons that would be present for the carbanion.
Show the orbital symmetry expected for the HOMO of the carbanion.
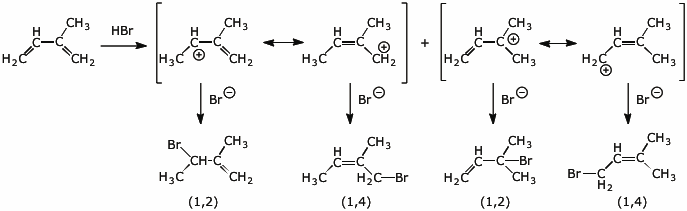
3. (4 points) Addition of HBr to the diene below gives a total of four products.
Show the two allylic cations (with both resonance forms for each) and the four possible products.
Identify each product as either a 1,2 or 1,4 addition.