Organic Chemistry II |
 |
|
Professor Carl C. Wamser |
||
Chem 335 - Winter Term |
Final Exam |
![]()
Organic Chemistry II |
 |
|
Professor Carl C. Wamser |
||
Chem 335 - Winter Term |
Final Exam |
![]()
1. (25 points) Write complete names for the following compounds, including stereochemistry if it is specifically shown.
a) 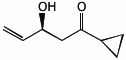
b) 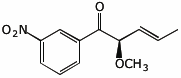
c) 
d) 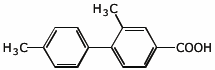
e) 
2. (15 points) Write complete structures for the following.
a) the LUMO of 1,3-butadiene
b) the Diels-Alder dimer of cyclopentadiene (endo isomer)
c) an aromatic ring with molecular formula C4H4N2
d) m-isobutylbenzyllithium
e) the ethyl hemiacetal of acetophenone
3. (15 pts) Arrange the following in order with respect to the property indicated.
Write MOST and LEAST under the compounds with the highest and lowest values, respectively.
a) reactivity in nitration
![]()
b) reactivity in nucleophilic substitution
![]()
c) basicity
![]()
d) acidity
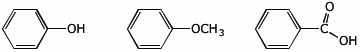
e) reactivity towards nucleophiles
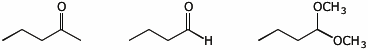
4. (15 pts) Complete each of the following reactions by adding the missing part:
either the starting materials, the necessary reagents and conditions, or the expected major product.
a) 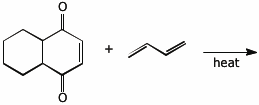
b) ![]()
c) 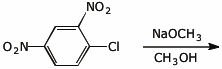
d) 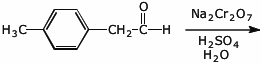
e) 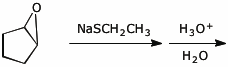
5. (15 pts) Complete each of the following reactions by adding the missing part:
either the starting materials, the necessary reagents and conditions, or the expected major product.
a) 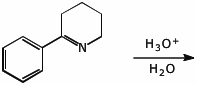
b) 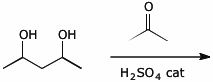
c) 
d) ![]()
e) 
6. (15 points) Write a complete mechanism for the reaction shown below.
Show all steps in the mechanism and all resonance forms for any intermediates.
Electron-pushing arrows are optional.
Identify the 1,2-products(s) and the 1,4-product(s).
Identify the product of kinetic control and the product of thermodynamic control.
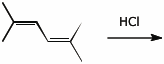
7. (15 points) Write a complete mechanism for the reaction shown below.
Show all steps in the mechanism and all resonance forms for any intermediates.
Electron-pushing arrows are optional.
![]()
8. (15 points) Write a complete mechanism for the reaction shown below.
Show all steps in the mechanism and all resonance forms for any intermediates.
Electron-pushing arrows are optional.
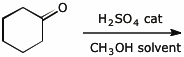
9. (20 points) Write complete mechanisms for the two cyclization reactions shown below. Show all steps in the mechanism and all resonance forms for any intermediates.
Use electron-pushing arrows.
a) 
b) 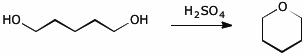
10. (30 points) Show how you would prepare each of the following target compounds.
All carbons in your final products must originate from benzene and/or any alcohol with four or fewer carbons.
a) 
b) 
c) 
11. (20 points) Identify the unknown compounds based on the information provided. Identify the structural features in your molecule that correlate with each of the spectral features.
a) Compound X has the molecular formula C10H14O2
IR shows a broad absorption at about 3300 cm-1 and no peaks in the 1700-1800 cm-1 range
Proton NMR shows the following peaks:
1.0 ppm, 3H, triplet
1.8 ppm, 2H, quartet
2.2 ppm, 1H, singlet
2.6 ppm, 1H, singlet
3.5 ppm, 2H, singlet
7.5 ppm, 5H, multiplet
b) Compound Y has the molecular formula C6H12O2
IR shows no absorptions above 3000 cm-1 and no peaks in the 1700-1800 cm-1 range
Proton NMR shows only two singlets with equal intensity at 2.0 ppm and 3.5 ppm.
Carbon-13 NMR shows four peaks at 25, 60, 110, and 140 ppm.