Organic Chemistry II |
 |
|
Professor Carl C. Wamser |
||
Chem 335 - Winter Term |
Exam 2 |
![]()
Organic Chemistry II |
 |
|
Professor Carl C. Wamser |
||
Chem 335 - Winter Term |
Exam 2 |
![]()
1. (15 points) Write accurate structures for each of the following
a) cis-3-methoxycyclopentanethiol
b) a p-methylbenzyl Grignard reagent
c) a C8H18 compound that would give only a singlet in its proton NMR spectrum
d) the olefin metathesis product from cyclopentene
e) (1R,2S)-1-methyl-1,2-cyclopentanediol
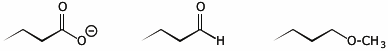
2. (15 pts) Arrange the following in order with respect to the property indicated. Write MOST under the compound with the highest value and LEAST under the compound with the lowest value.
a) acidity
![]()
b) basicity
![]()
c) nucleophilicity
![]()
d) number of peaks in its C-13 NMR spectrum
![]()
e) reactivity towards LiAlH4
3. (15 points) Complete each of the following reactions by adding electron-pushing arrows to arrive at the indicated products. Only add the arrows.
a) 
b) ![]()
c) 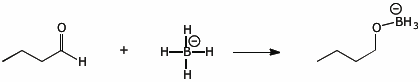
d) 
e) 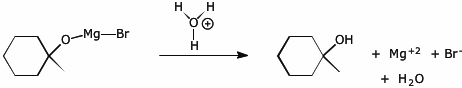
4. (15 pts) Complete each of the following reactions by adding the necessary reagents and conditions.
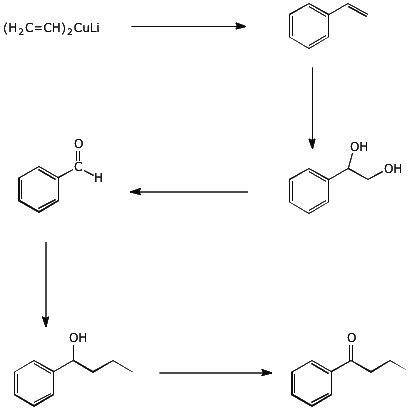
5. (20 points) Write synthetic sequences that show how you would prepare each of the following compounds. All the carbons in your final products must come from either benzene or any alcohol that has three or fewer carbons.

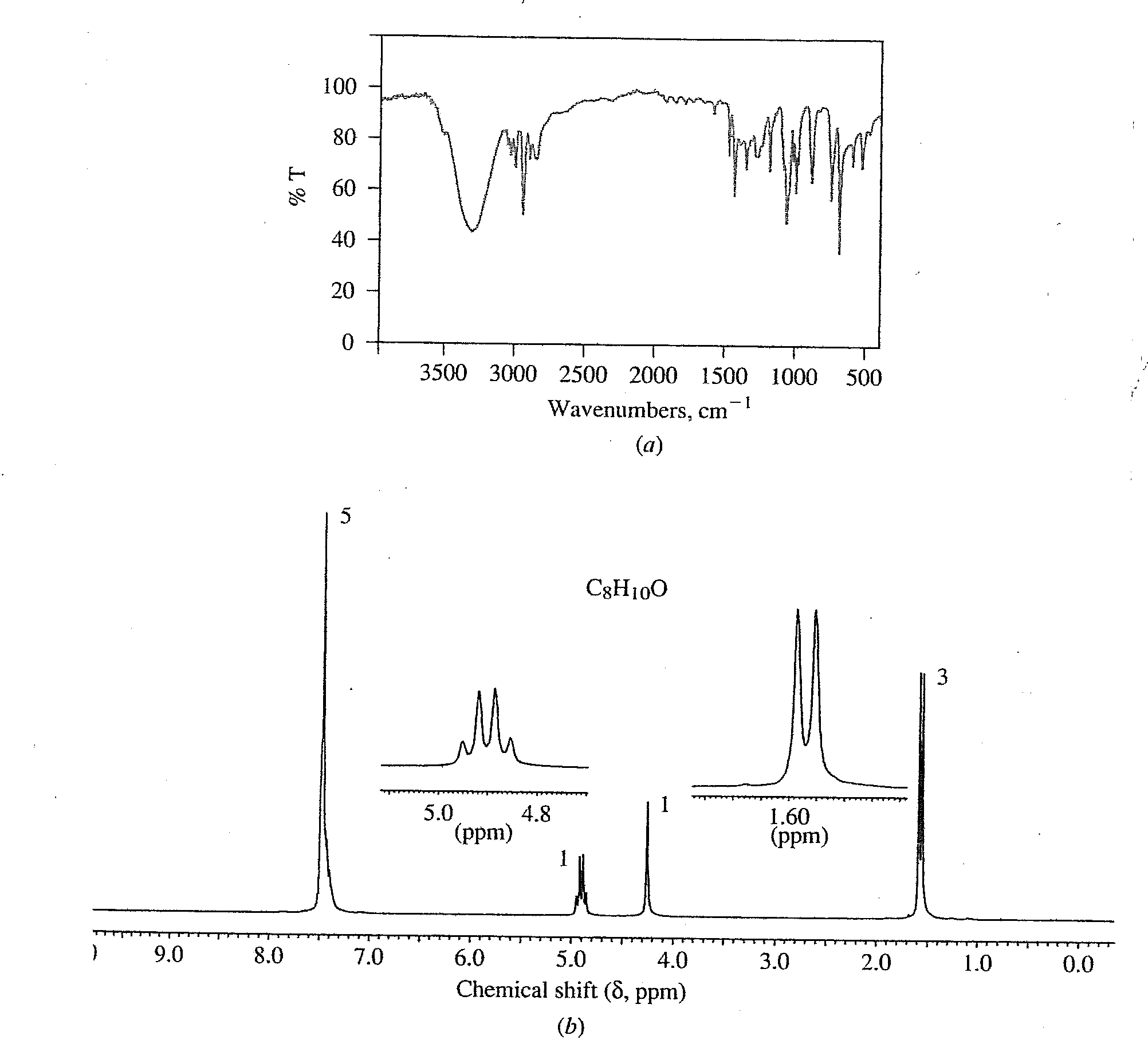
6. (10 points) Identify the unknown compound that shows the following spectral data.
Identify each H in the NMR spectrum and at least one feature of the IR spectrum.
7. (10 points) Identify the unknown compound that shows the following spectra data.
Correlate each of the spectral features below with a structural feature in your final compound.
Molecular formula C6H10
Proton NMR
2.5 ppm, 1H, singlet
2.0 ppm, 2H, doublet
1.7 ppm, 1H, multiplet
1.0 ppm, 6H, doublet
C-13 NMR
5 peaks at 80, 70, 40, 30, 20 ppm
IR
strong sharp peaks at 3315 and 2180 cm-1