Organic Chemistry II |
 |
|
Professor Carl C. Wamser |
||
Chem 335 - Winter Term |
Exam 1 |
![]()
Organic Chemistry II |
 |
|
Professor Carl C. Wamser |
||
Chem 335 - Winter Term |
Exam 1 |
![]()
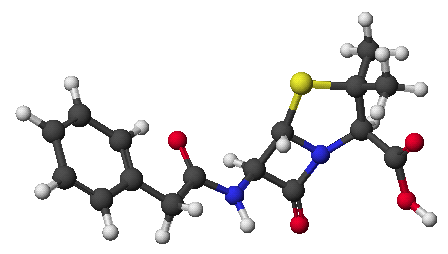
1. (15 points) Write complete names for the following.
a) 
b) 
c) 
2. (15 points) Write complete structures for the following.
a) 3-pyridinecarboxylic acid
b) the LUMO of the allyl anion
c) o-isobutylbenzyl alcohol
d) 1-methoxy-5-nitronaphthalene
e) the Diels-Alder product from 1,3-cyclopentadiene and ethyne
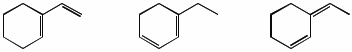
3. (15 points) Arrange the following in order with respect to the property indicated. Write “MOST” under the compound with the highest value and “LEAST” under the compound with the lowest value.
a) reactivity in bromination

b) reactivity in a Diels-Alder reaction
c) reactivity in nucleophilic substitution
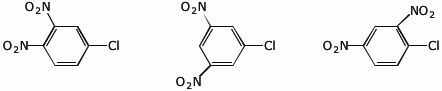
d) reactivity in nitration

e) fractional amount of para-substitution expected
![]()
4. (15 pts) Complete each of the following reactions by adding the expected major product or the necessary reagents and conditions.
a) ![]()
b) ![]()
c) 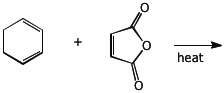
d) 
e) 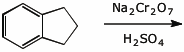
5. (15 points) Write a complete mechanism for the Friedel-Crafts reaction shown below.
Show all steps and all resonance forms for the intermediate involved. Just form the para product.

6. (10 points) Show a reaction sequence that could be used to prepare the following compound starting from benzene plus any other needed compounds or reagents.

7. (15 points) The diene below undergoes both 1,2 and 1,4 addition of HBr.
There are two possible allylic cations (A and B), each with two resonance forms, and there are four possible addition products (C, D, E, F).
Write the cations with all resonance forms.
Write all the products, including designating which are 1,2 and which are 1,4 products.
Predict the kinetically controlled product and the thermodynamically controlled product.
![]()