|
|
Organic
Chemistry II
|
|
Professor
Carl C. Wamser
|
|
|
Chapter
16 Notes
|

Ethers, Epoxides, Sulfides

Structure and Properties
- C-O-C bond
- bent bond, slightly polar
- no H-bonding (low bp), but H-bond acceptor (slightly water-soluble)
- crown ethers - specific complexing agents for cations
Nomenclature
- IUPAC: alkoxy- substituent
- common: alkyl ether
- diethyl ether = ethyl ether = "ether"
- cyclic ethers have special names (and lots of different names)
- epoxides (3-membered rings) :
oxirane, epoxyalkane, alkene oxide
- oxetane (4), oxoloane (5), oxane (6)
- sulfides ( C-S-C bond )
- IUPAC uses alkylthio- substituent
- common uses alkyl sulfide
- disulfides: ( C-S-S-C bond )
Preparations of Ethers
- Williamson synthesis
- SN2 reaction of alkoxide with alkyl halide
- alkyl halide should be CH3 or 1°
- dehydration of alcohols
- for symmetrical ethers only, best with CH3 or
1°
Preparations of Sulfides
- Williamson synthesis with thiolate anion + alkyl halide
- double displacement with Na2S and alkyl halide
(symmetrical sulfides only)
Reactions of Ethers
- generally unreactive towards oxidation, reduction, base, and most acids
(make good solvents)
EXCEPT for
- cleavage by conc. HI or HBr
R-O-R + 2 HX ---> 2 R-X + H2O
reaction is either SN1 or SN2
(or both), depending on the R groups
- radical autoxidation to form hydroperoxides
Reactions of Sulfides
- similar to ethers except S is a better nucleophile and leaving group
and S is easily oxidized
- sulfoxide ( S=O bond ) , sulfone ( two S=O bonds )
- sulfonium ions (trivalent S+)
Preparations of Epoxides
- oxidation of alkenes by peroxyacids
- internal SN2 in halohydrins
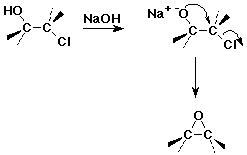
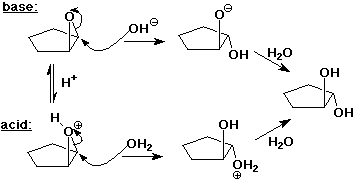
Reactions of Epoxides
- unlike most other ethers, epoxides are reactive, because of ring strain
- acid-catalyzed ring opening
- protonation makes ring opening easier
- always backside attack by nucleophile (like SN2)
- but nucleophile attacks more substituted carbon (like SN1)
- nucleophilic ring opening
- direct attack without protonation requires a strong nucleophile
- backside attack on less-hindered carbon (like SN2)
![]()

![]()
![]()
