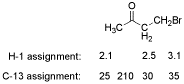Organic Chemistry II |
 |
|
Professor Carl C. Wamser |
||
Chem 335 - Winter Term |
Quiz 13 |
![]()
Organic Chemistry II |
 |
|
Professor Carl C. Wamser |
||
Chem 335 - Winter Term |
Quiz 13 |
![]()
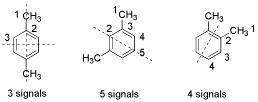
1. (3 points) The ortho, meta, and para isomers of the xylenes (dimethylbenzenes) are easily distinguished by C-13 NMR. Explain.
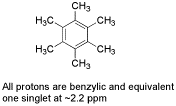
2. (2 points) Identify the compound of formula C12H18 that would have only one singlet in its H-1 NMR (at 2.2 ppm).
3. (5 points) Identify the unknown compound X based on the following.
For
each piece of data, indicate what structural conclusions you can draw.
Assign
individual peaks in the NMR spectra to specific C’s and H’s.
(s = singlet,
d = doublet, t = triplet, q = quartet)
Molecular formula: C4H7BrO IHD =
Mass spectrum: approximately equal peaks at 150 and 152
IR spectrum: strong peak at 1720 cm-1
C-13 NMR spectrum: peaks (ppm) at 25 (q), 30 (t), 35 (t), 210 (s)
H-1 NMR spectrum: peaks (ppm) at 2.1 (s, 3H), 2.5 (t, 2H), 3.1 (t, 2H)