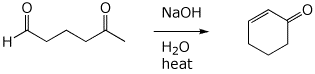Organic Chemistry II |
 |
|
Professor Carl C. Wamser |
||
Chem 335 - Winter Term |
Exam 3 Answer Key |
![]()
Organic Chemistry II |
 |
|
Professor Carl C. Wamser |
||
Chem 335 - Winter Term |
Exam 3 Answer Key |
![]()
1. (15 points) Write complete names for the following compounds, including stereochemistry if it is specifically shown.
a) 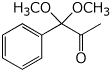
b) 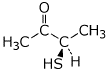
c) 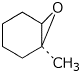
d) ![]()
e) 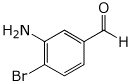
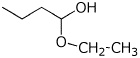
2. (15 points) Write complete structures for the following.
a) benzophenone oxime
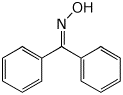
b) the ethyl hemiacetal of butanal
c) the best resonance form of the enolate anion of cyclopentanone
![]()
d) m-(ethylthio)benzenethiol
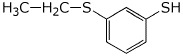
e) (R)-1,2-epoxypentane
![]()
3. (10 pts) All of the following are simple ACID-BASE reactions (just add or remove a proton). Write all the resonance forms for the conjugate acid or conjugate base that would be formed in each case.
a) 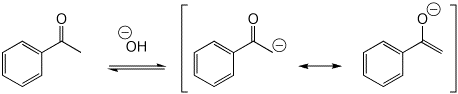
b) 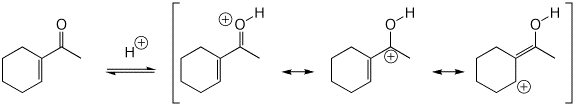
c) 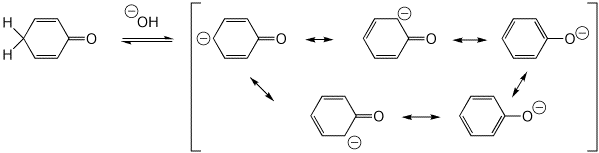
4. (15 pts) Complete each of the following reactions by adding the missing part: either the starting materials, the necessary reagents and conditions, or the expected major product.
a) 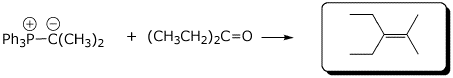
b) 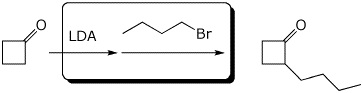
c) 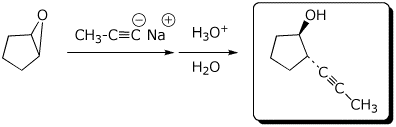
d) 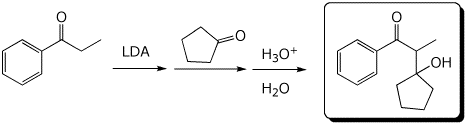
e) 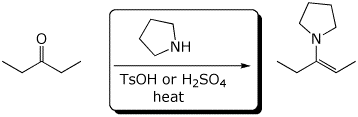
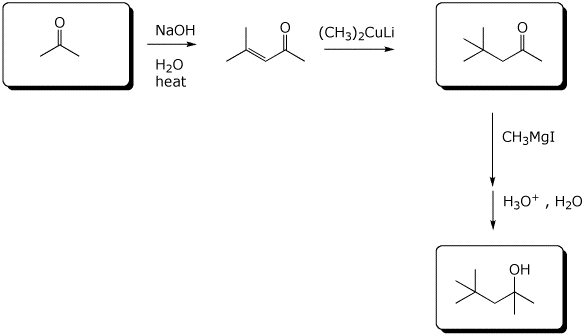
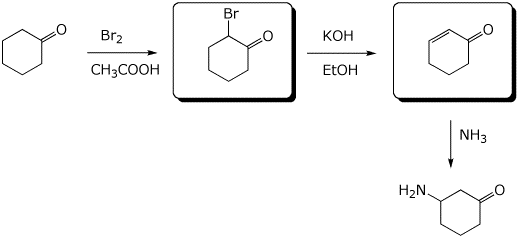
5. (10 points) Complete the following synthetic sequences by filling in the empty boxes.
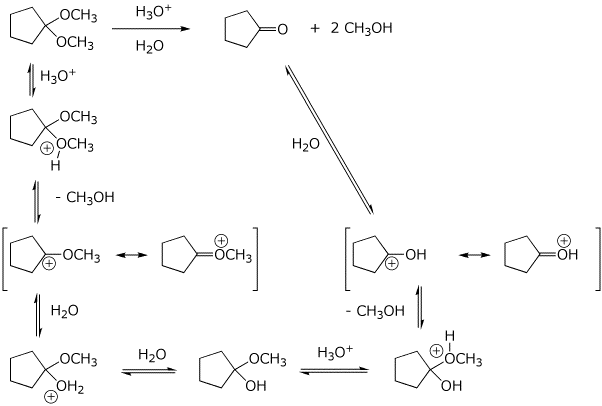
6. (20 points) Write a complete mechanism for the acid-catalyzed reaction shown below.
Show all steps in the mechanism and all resonance forms for any intermediates.
Use the correct forms for the predominant acid and base in the system, i.e., do not use H+ and - H+.
Electron-pushing arrows are optional.
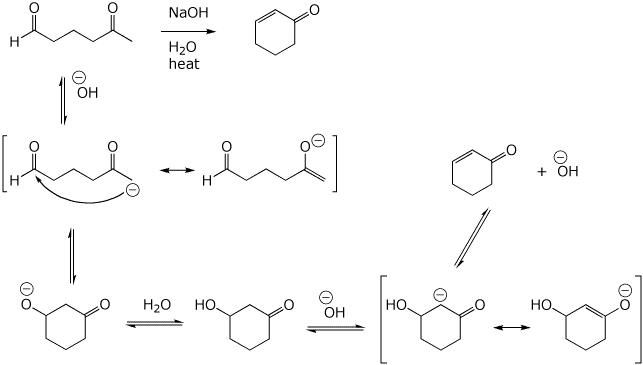
7. (15 points) Write a complete mechanism for the base-catalyzed reaction shown below.
Show all steps in the mechanism and all resonance forms for any intermediates.
Use the correct forms for the predominant acid and base in the system, i.e., do not use H+ and - H+.
Electron-pushing arrows are optional.