Organic Chemistry II |
 |
|
Professor Carl C. Wamser |
||
Chem 335 - Winter Term |
Exam 2 |
![]()
Organic Chemistry II |
 |
|
Professor Carl C. Wamser |
||
Chem 335 - Winter Term |
Exam 2 |
![]()
1. (15 points) Write complete names for the following compounds, including stereochemistry if it is specifically shown.
a) 
b) 
c) ![]()
d) ![]()
e) ![]()
2. (15 points) Write complete structures for the following.
a) the cyclic osmate ester from cyclohexene
b) 2-mercaptoethanol
c) a neutral compound with 5 carbons that would show only one C13 NMR peak
d) an ethyl chromate ester
e) a nickel compound that satisfies the 18-electron rule using only CO ligands
3. (10 pts) Balance the following redox reaction.

4. (15 pts) Complete each of the following reactions by adding the expected major product or the necessary reagents and conditions.
a) 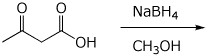
b) 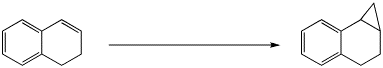
c) 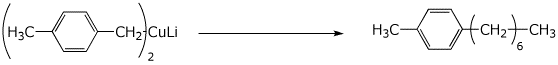
d) 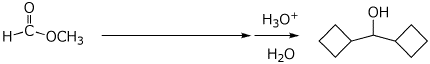
e) 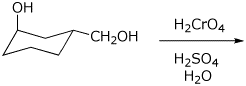
5. (15 points) Describe the data that you would expect for the compound below.

a) IHD =
b) The mass spectrum shows large M+ and (M-CH3) peaks.
Calculate
the (m/z) values for these two peaks and give a likely structure for the
(M-CH3) fragment.
c) Indicate where you would expect to see C-H stretching frequencies. Indicate at least one prominent peak expected in the multiple bond stretching region.
d) How many different absorptions would be expected in the C13 NMR spectrum?
e) Give a complete description of the expected
H NMR spectrum, using the standard format as shown on page 7.
(for each
absorption, indicate expected chemical shift range, integration intensity,
and splitting pattern)
6. (15 points) The three isomeric alcohols below are synthetic targets to be made by Grignard coupling reactions. The possible Grignard reagents to be used could be prepared from any of the three bromides shown.

For ALL THREE target molecules, indicate the final C-C bond-forming reaction for each case.
For ANY ONE of the three targets, write out a complete synthesis. All carbons in the final target must originate from either benzene, ethylene oxide, or an alcohol having three or fewer carbons.
7. (15 points) Identify the unknown compound based on the data given below. For each item of data, describe what structural information you can deduce.
Molecular formula C10H14
C13 NMR shows 7 absorptions, 4 in the 110-175 ppm region, and 3 in the 0-50 ppm region
IR shows C-H stretching just above and just below 3000 cm-1
H NMR spectrum:
1.5 ppm, 3H, triplet
2.4 ppm, 6H, singlet
2.6 ppm, 2H, quartet
7.5 ppm, 1H, singlet
7.8 ppm, 2H, singlet