Organic Chemistry II |
 |
|
Professor Carl C. Wamser |
||
Chem 335 - Winter Term |
Exam 1 |
![]()
Organic Chemistry II |
 |
|
Professor Carl C. Wamser |
||
Chem 335 - Winter Term |
Exam 1 |
![]()
1. (15 points) Write complete structures for the following.
a) m-bromobenzyl chloride
b) 1-chloro-7-nitronaphthalene
c) cycloheptatrienyl cation
d) the Diels-Alder dimer of cyclopentadiene (endo isomer)
e) 1,3-dimethylpyrrole
2. (20 pts) Write all of the resonance forms for the intermediates that would be formed in each reaction below.
(Donít finish the reaction, just write the intermediate formed directly from the reaction described.)
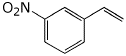
a) para attack by Br+
![]()
b) SN1 ionization to a carbocation
![]()
c) deprotonation of the O-H bond
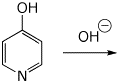
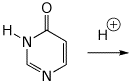
d) protonation on O
3. (15 pts) Complete each of the following reactions by adding the expected major product.
a) 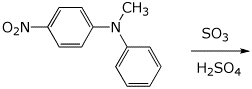
b) 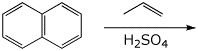
c) 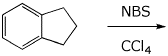
d) 
e)
4. (a) (10 points) Consider the conjugated, delocalized pentadienyl anion that would be obtained by removing a proton from 1,3-pentadiene.
Write the expected pattern of energy levels for the molecular orbitals, including the number of electrons in each orbital.
Show the orbital symmetry of the HOMO.
4.(b) (10 points) Consider the conjugated, delocalized cyclopentadienyl anion that would be obtained by removing a proton from 1,3-cyclopentadiene.
Write the expected pattern of energy levels for the molecular orbitals, including the number of electrons in each orbital.
Show the orbital symmetry of the HOMO (either one).
5. (15 points) Starting from the diene shown below, illustrate the possible pathways for addition of HBr.
Write two different allylic carbocations with both resonance forms for each.
Write the possible products and identify each one as a 1,2 or 1,4 product.
Predict the more stable carbocation and the most stable product.
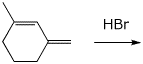
6. (15 points) Show a reaction sequence that could be used to prepare the following compound starting from benzene. Use NBS at one point in your synthesis.