Organic Chemistry II |
 |
|
Professor Carl C. Wamser |
||
Chem 335 - Winter 2009 |
Quiz 13 |
![]()
Organic Chemistry II |
 |
|
Professor Carl C. Wamser |
||
Chem 335 - Winter 2009 |
Quiz 13 |
![]()
1. (1 pt) What bond would be responsible for the highest frequency stretching vibration in the molecule shown below?
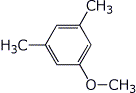
2. (1 pt) How many C-13 absorptions would be expected for the compound below?
 6
6 3. (1 pt) At what m/z values would the M and M+2 peaks appear for the following?
4. (1 pt) Which compound would show the longest wavelength absorption in uv-vis?
![]()
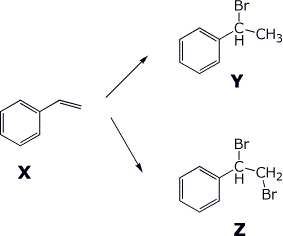
5. (6 pts) Compound X (C8H8) adds one equivalent of HBr to give compound Y.
Compound
X adds one equivalent of Br2 to give compound Z.
Identify compounds
X, Y, and Z.
Assign the NMR absorptions to specific hydrogens.
Compound Y proton
NMR spectrum:
7.5 ppm, 5H, multiplet
3.7 ppm, 1H, quartet
1.9 ppm, 3H, doublet
Compound Z proton NMR spectrum:
7.5 ppm, 5H, multiplet
3.8 ppm, 1H, triplet
3.2 ppm, 2H, doublet