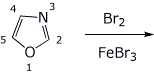Organic Chemistry II |
 |
|
Professor Carl C. Wamser |
||
Chem 335 - Winter 2009 |
Exam 1 Answer Key |
![]()
Organic Chemistry II |
 |
|
Professor Carl C. Wamser |
||
Chem 335 - Winter 2009 |
Exam 1 Answer Key |
![]()
1. (21 pts) Write accurate structures that would clearly illustrate the following:
a) m-methoxybenzyl alcohol

b) 2-chloro-4-nitroaniline

c) the HOMO for s-trans-2,4-hexadiene
![]()
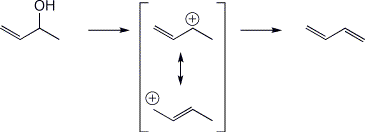
d) the two resonance forms of the allylic cation from dehydration of 3-buten-2-ol
e) the Diels-Alder product of cyclopentadiene with itself (a dimer)

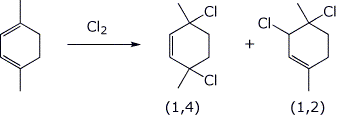
f) the conjugate addition product from addition of chlorine to 1,4-dimethyl-1,3-cyclohexadiene
g) an aromatic carbanion
![]()
2. (15 pts) Arrange the following in order with respect to the property indicated. Write MOST and LEAST under the compounds with the highest and lowest values, respectively.
a) stability
![]()
b) reactivity in electrophilic aromatic substitution
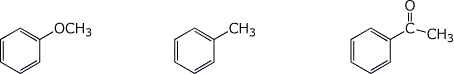
c) SN1 reactivity
![]()
d) expected percentage of ortho product
![]()
e) reactivity towards bromine
![]()
3. (15 pts) Complete each of the following reactions by adding the expected major product.
a) 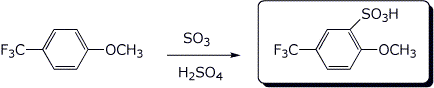
b) 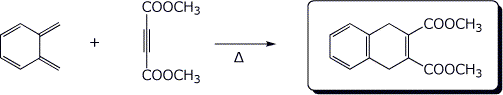
c) 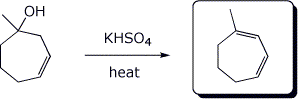
d) 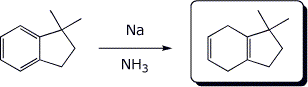
e) 
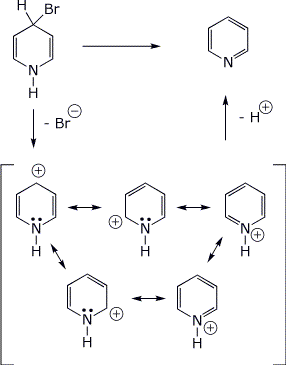
4. (10 pts) The compound shown below undergoes very rapid E1 elimination to form pyridine.
Show the expected cation intermediate in this reaction including all resonance forms.
5. (21 pts) The diene shown below could undergo addition of HI to give two possible allylic cations (A and B). Each cation could react to give two possible iodide addition products (either 1,2 or 1,4).
Show structures for A and B (each with their resonance forms) and the products that would be expected from each cation. Ignore stereochemistry issues.
Predict which of the two cations (A or B) would be more stable.
Identify the kinetic and thermodynamic product from each cation.
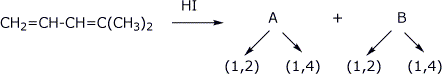

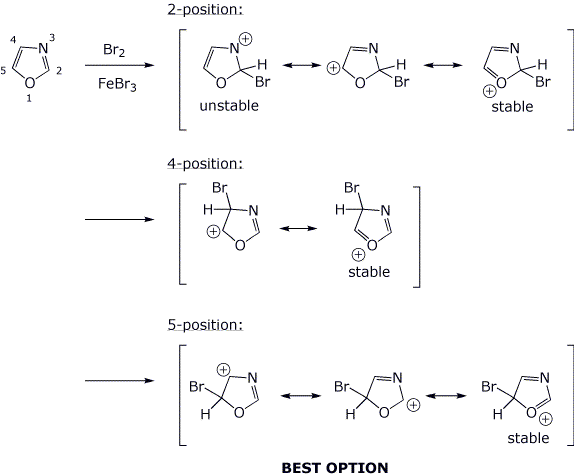
6. (18 pts) The compound shown below could conceivably undergo electrophilic substitution at the 2, 4, or 5 position.
For each of the three cases, write all the resonance forms for the expected bromination intermediate and predict the most favorable substitution site.