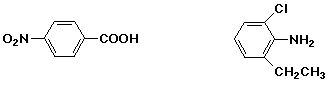|
|
Organic
Chemistry II
|
|
Professor
Carl C. Wamser
|
|
|
Chapter
11 Notes
|

Aromaticity

Aromaticity
- pleasant odor
- unusually low reactivity
- substitution, not addition
- unusually stable
- characteristic ring structure with delocalized pi bonding
- NMR ring current effect
Benzene - stability
- C6H6 (1,3,5-cyclohexatriene
?)
- no typical C=C reactions
unreactive with HX, X2, KMnO4
- reaction requires extreme conditions
when reaction does occur, it is substitution not addition
- Heats of hydrogenation:
cyclohexene + H2 -28.6 kcal/mol (typical pi bond)
1,3-cyclohexadiene + 2 H2 -55.8 kcal/mol
(more stable than 2 pi bonds by 1.4 kcal/mol - conjugation)
benzene + 3 H2 -49.8 kcal/mol
(more stable than 3 pi bonds by 36 kcal/mol - aromaticity)
Benzene - structure
- all C are sp2 (trigonal, 120° angles)
ideal for a planar hexagon

- all C-C bonds are the same (139 pm)
- compare C-C (154 pm), C=C (134 pm)
- cyclic conjugated pi bonds are unusually stable (resonance)
Nomenclature of Aromatics
- monosubstituted benzenes:
there are many common names
- disubstituted benzenes:
ortho (1,2-), meta (1,3-), para (1,4-)
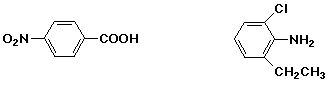
p-nitrobenzoic acid / / / 2-chloro-6-ethylaniline
Nomenclature of Aromatics
- group names:
phenyl C6H5
benzyl C6H5CH2

(E)-1-phenyl-1-butene
Spectroscopy
- NMR - ring current effect: aryl Hs are deshielded
annulenes - outside Hs are deshielded, inside Hs are strongly shielded ( < 0
ppm)
- IR - typical C=C stretching and C-H bends depending on substitution
patterns
Benzenoid Hydrocarbons
- fused six-membered rings
- know naphathlene, anthracene, phenanthrene
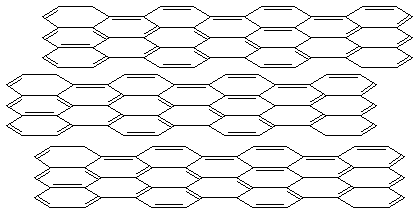
Graphite
- extended sheets of benzene rings
electrically conductive, good lubricant
Fullerenes
- curved closed form of 60 carbon atoms in benzene rings with intervening
5-membered rings (soccer ball pattern)
- subject of the 1996 Nobel Prize in chemistry
Annulenes
- fully conjugated rings
e.g., cyclobutadiene, cyclooctatetraene (nonaromatic)
Nonbenzenoid Hydrocarbons
- ring sizes other than six
e.g., azulene
Aromatic Heterocycles
- six-membered rings - N replaces C
e.g., pyridine, pyrimidine
- five-membered rings - lone pair on N, O, or S replaces a pi bond
e.g., pyrrole, furan, thiophene, imidazole
Molecular Orbitals
- combination of n atomic orbitals (AOs) must give n molecular orbitals
(MOs)
- six pi MOs for benzene (see text pictures)
Huckel's Rule - Aromaticity
- a compound will display aromaticity if
* it is a continuous cyclic array of p orbitals
* it is planar
* it has a total number of pi electrons of 2, 6, 10, 14, ... (4n+2)
- pi systems that are planar and cyclic but have 4n electrons are antiaromatic
- cyclobutadiene (rectangular - like two separate double bonds)
- cyclooctatetraene (tub-shaped - like four separate double bonds)
Aromatic Ions
- cyclopentadienyl anion (5 p orbitals but 6 pi electrons - aromatic)
makes a good ligand for metal complexation C5H5 -1
e.g., sandwich compounds like ferrocene Fe(Cp)2
- cyclooctatetraenyl dianion (8 p orbitals and 10 pi electrons - aromatic)
makes a good ligand for large metals C8H8 -2
e.g., sandwich compounds like uranocene U(COT)2
- cyclopropenyl cation C3H3+
, cycloheptatrienyl cation (tropylium ion) C7H7+
Reduction Reactions
- Birch reduction - forms 1,4-cyclohexadiene
Reactions at Benzylic Positions
- anion, cation, or radical at a benzylic position can be stabilized
by resonance delocalization
- SN1 and SN2 reactions are favorable at benzylic positions
- alkylaromatics are easily oxidized to benzoic acid
- halogenation of alkylaromatics is preferred at the alpha (benzylic)
position
- arylalkenes (conjugated) are easily formed by elimination
- arylalkenes that can make benzylic intermediates undergo rapid addition
reactions
![]()

![]()
![]()