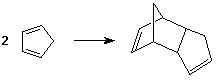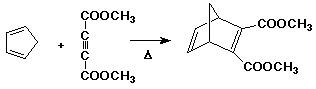|
|
Organic Chemistry
II
|
|
Professor Carl C. Wamser
|
|
|
Chapter 10 Notes
|

Conjugation

Molecular Orbitals (MOs) of Conjugated Pi Systems
- n atomic orbitals (AOs) combine to make n molecular orbitals (MOs)
- ethene - pi bond and pi* antibond, with two electrons in the pi bond
- allyl pi system (could be cation, radical, or anion) - 3 pi MOs (bonding,
nonbonding, antibonding)
- allyl cation (2 pi electrons), allyl radical (3 pi electrons),
allyl anion (4 pi electrons)
- 1,3-butadiene - 4 pi MOs (two bonding and two antibonding) with 4 pi
electrons
- frontier MOs are the most important for electron pushing
- highest occupied MO - HOMO
- lowest unoccupied MO - LUMO
Allylic Systems
- relative stability of cation, radical, anion
- allylic bromination with NBS
- reactivity in SN1 and SN2 reactions
- allylic rearrangements
Categories of Dienes
- cumulated (allenes)
- conjugated (most stable)
- isolated
- s-trans and s-cis conformers
Conjugate Additions
- conjugated dienes are very reactive towards electrophiles
- addition gives a relatively stable allylic cation
- conjugated dienes can give 1,2 or 1,4 addition
- the two ends of the allyl cation may be nonequivalent

- addition at low temperature favors the 1,2-addition product (kinetically
controlled)
- addition at high temperature favors the 1,4-addition product (thermodynamically
controlled)
- heating the kinetic mixture of products gives the thermodynamic mixture
(i.e., mostly 1,4)
- kinetic control (lower Ea) - the more substituted cation has
more + charge, reacts faster
- thermodynamic control - the more substituted alkene is the more
stable product
- thermodynamic control requires equilibration to form the more
stable product, since it has a higher barrier
- Note - this situation of the more stable product having a higher activation
barrier is unusual
- in most situations, a more stable product means a lower barrier
(The Hammond Postulate)
The Diels-Alder Reaction
- cycloaddition of a diene to an alkene (usually called a dienophile
in this reaction)
- overall, two pi bonds are converted to two sigma bonds while making
the ring
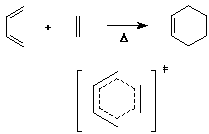
- note that the transition state is a 6-membered ring of delocalized
pi electrons
- such transition states are particularly stable when they have
(4n+2) electrons
- the diene must be in an s-cis conformation for this reaction
- s-cis means the single bond between the two double bonds is cis
- cyclic dienes are much more reactive than acyclic dienes
- cyclopentadiene undergoes reaction with itself
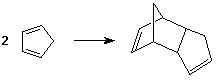
- the reaction goes best with alkenes having electron-withdrawing substituents
(dienophiles)
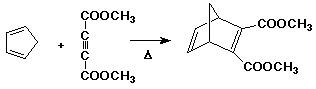
- this allows good electron flow from the diene HOMO to the alkene
LUMO
- Lewis acids also enhance the Diels-Alder reaction by complexing the
dienophile
- the combination of electron-deficient diene with electron-rich alkene
also works
- stereochemistry of the diene and the alkene is retained
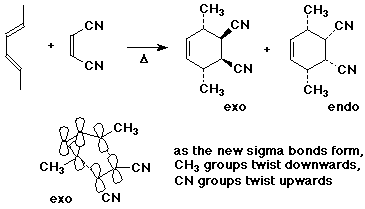
- endo product is usually the major product, due to secondary interactions
between the pi systems

![]()

![]()
![]()