Organic Chemistry II |
 |
|
Professor Carl C. Wamser |
||
Chem 335 - Winter 2008 |
Quiz 18 |
![]()
Organic Chemistry II |
 |
|
Professor Carl C. Wamser |
||
Chem 335 - Winter 2008 |
Quiz 18 |
![]()
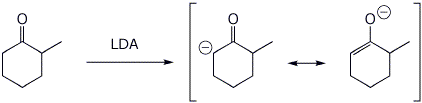
1. (3 points) Write the structure of the enolate (both resonance forms) expected to be formed when LDA reacts with 2-methylcyclohexanone. Write the structure of LDA.
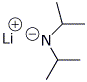
2. (2 points) Complete each of the following two reactions:
a) 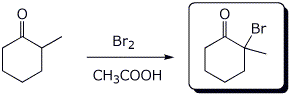
b)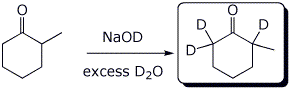
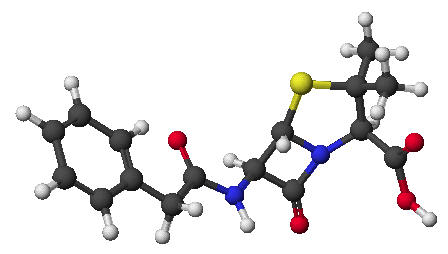
3. (2 points) Draw the structures of the two compounds that would have reacted in an aldol condensation (with dehydration) to form the product shown below.
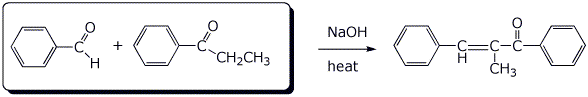
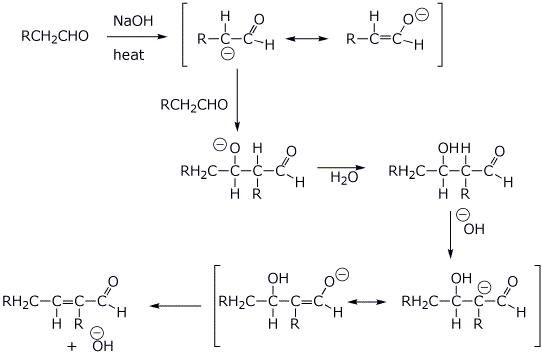
4. (3 points) Write a complete mechanism for the aldol condensation (with dehydration) of the generic aldehyde shown below. Show all steps and show all resonance forms for any intermediates.