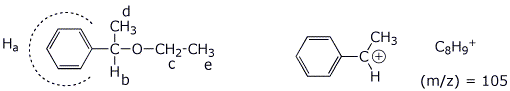Organic Chemistry II |
 |
|
Professor Carl C. Wamser |
||
Chem 335 - Winter 2008 |
Quiz 13 |
![]()
Organic Chemistry II |
 |
|
Professor Carl C. Wamser |
||
Chem 335 - Winter 2008 |
Quiz 13 |
![]()
1. (2 points) Give the molecular formula and the index of hydrogen deficiency (IHD) for benzene. For the value you calculate, explain how the molecular structure fits that value.
2. (2 points) Describe the expected H NMR and C NMR spectra for benzene, including the expected chemical shift range and the multiplicity of each peak (do not assume there has been proton decoupling in the spectra).
3. (6 points) Identify the unknown compound based on the following
data. For each item of spectroscopic information, indicate what structural
conclusion you can draw.
Identify each of the lettered absorptions with the hydrogens in the final structure.
Molecular
formula:
C10H14O Calculated
IHD = ____4____
IR spectrum:
no
significant peaks higher than 3100 cm-1 __no
O-H bonds__
no
significant peaks between 1700 and 2700 cm-1 __no
C=O bonds_
H
NMR spectrum:
a 7.1
ppm, 5H, multiplet
b 3.7
ppm, 1H, quartet
c 3.4
ppm, 2H, quartet
d 1.9
ppm, 3H, doublet
e 1.6
ppm, 3H, triplet
Mass spectrum:
parent
molecular ion at (m/z) = 150 _molec.
weight = 150_
large
peak at (m/z) = 105 _stable
benzylic cation (see below)_