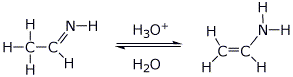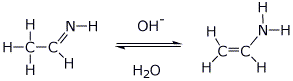Organic Chemistry II |
 |
|
Professor Carl C. Wamser |
||
Chem 335 - Winter 2008 |
Final Exam |
![]()
Organic Chemistry II |
 |
|
Professor Carl C. Wamser |
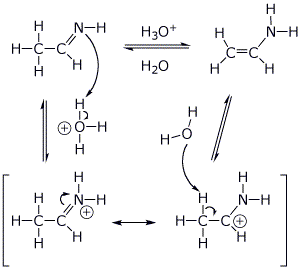
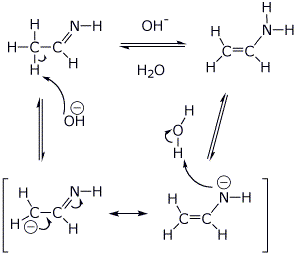
||
Chem 335 - Winter 2008 |
Final Exam |
![]()
1. (25 points) Write complete names for each of the following:
a) 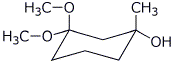
b) 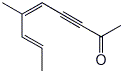
c) 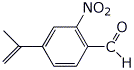
d) 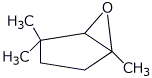
e) 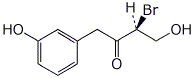
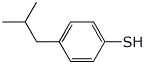
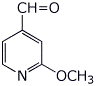
2. (15 points) Write clear structures for each of the following:
a) 4-isobutylbenzenethiol
b) 2-methoxypyridine-4-carbaldehyde
c) lithium aluminum hydride
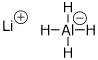
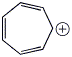
d) an aromatic cation with 4n+2 = 6
e) the HOMO of 1,3,5-hexatriene
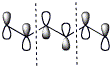
3. (15 points) Arrange the following in order with respect to the property
indicated.
Write MOST and LEAST under the compounds with the highest and
lowest values, respectively.
a) rate of reaction with bromine
MIDDLE / / / LEAST / / / MOST
b) rate of reaction with methanol
MIDDLE / / / MOST / / / LEAST
c) rate of reaction with nitric acid plus sulfuric acid
LEAST / / / MIDDLE / / / MOST
d) number of absorptions in the C-13 NMR spectrum
MIDDLE / / / MOST / / / LEAST
e) number of hydrogens exchanged for deuterium in NaOD/D2O
MOST / / / MIDDLE / / / LEAST
4. (15 points) Complete each of the following reactions by writing the structure of the expected product:
a) 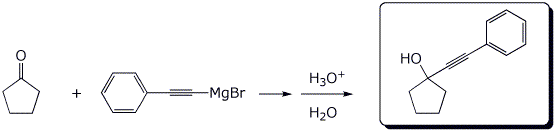
b) 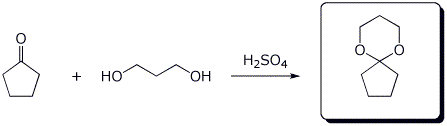
c) 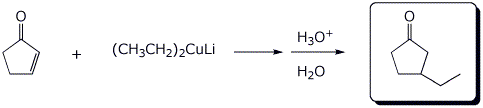
d) 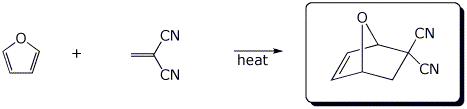
e) 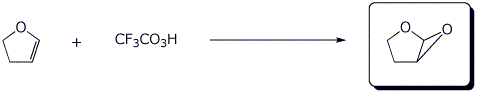
5. (15 points) Complete each of the following reactions by writing the structure of the expected product:
a) 
b) 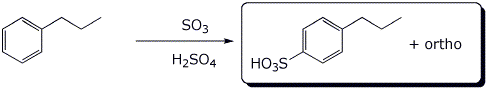
c) 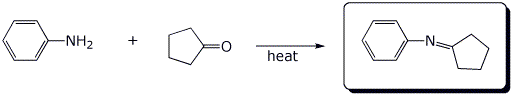
d) 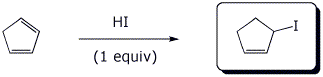
e) 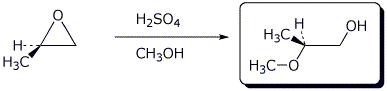
6. (20 points) Show how to prepare each of the compounds below. All the carbons in the products must originate from alcohols having four or fewer carbon atoms or from benzene. You may use any needed reagents or solvents.
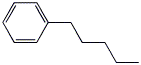
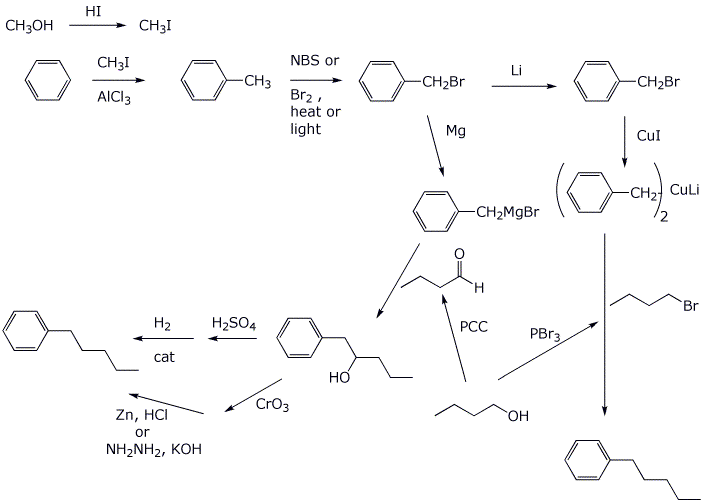
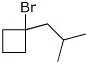
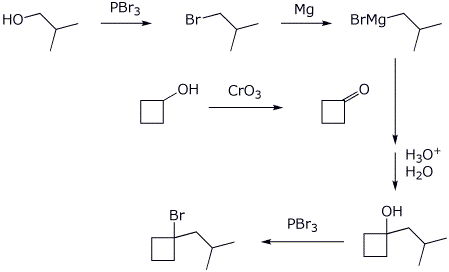
7. (15 points) Write a complete mechanism for the reaction shown below. Just show the pathway to the expected major product. Show all steps and all resonance forms for all intermediates involved.
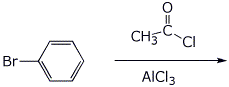
8. (20 points) Write a complete mechanism for the reaction shown below. Show all steps and all resonance forms for all intermediates involved.
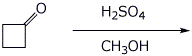
9. (15 points) Write a complete mechanism for the aldol condensation (with dehydration) of the compound shown below. Show all steps and all resonance forms for all intermediates involved.
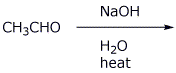
10. (15 points) Identify the unknown compound based on the data below. For each item of information, show what structural conclusions you draw, e.g., assign all the individual absorption peaks to specific parts of the molecule.
molecular formula C11H16O
IHD = _____4_____
IR:
strong broad absorption at 3300 cm-1
presence of O-H
no absorptions between 1680 and 2800 cm-1
absence of C=O and triple bonds
1H NMR:
a 7.8 ppm 5H multiplet
b 4.5 ppm 1H broad singlet
c 3.6 ppm 1H triplet
d 1.8 ppm 2H doublet of doublets
e 1.3 ppm 1H complex multiplet
f 1.0 ppm 6H doublet
11. (15 points) Identify the unknown compound based on the data below. For each item of information, show what structural conclusions you draw e.g., assign all the individual absorption peaks to specific parts of the molecule.
molecular formula C8H12O2
IHD = _____3______
IR:
strong absorption at 1730 cm-1
presence of C=O
no absorptions above 3200 cm-1
absence of O-H
1H NMR:
a 6.2 ppm 1H singlet
b 2.2 ppm 2H quartet
c 1.0 ppm 3H triplet
12. (15 points) Imines and enamines are tautomers analogous to the keto-enol tautomerism. Use the case below to illustrate the interconversion of an imine and enamine in acid or base. The example is very simple so that you should show every atom and all electron-pushing arrows throughout your mechanisms (including for resonance forms).