Organic Chemistry II |
 |
|
Professor Carl C. Wamser |
||
Chem 335 - Winter 2008 |
Exam 3 |
![]()
Organic Chemistry II |
 |
|
Professor Carl C. Wamser |
||
Chem 335 - Winter 2008 |
Exam 3 |
![]()
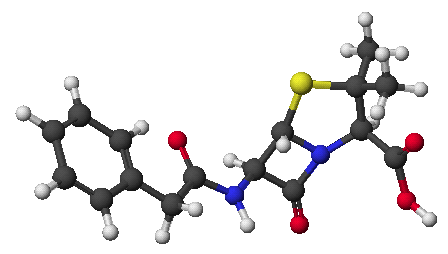
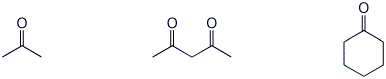
1. (15 points) Write complete names for each of the following:
a) 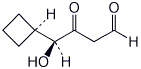
b) 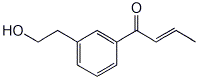
c) 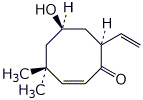
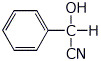
2. (15 points) Write clear structures for each of the following:
a) the cyanohydrin of benzaldehyde
b) the most stable enol of pentane-2,3-dione
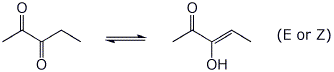
c) a compound of formula C5H10O that would give a positive iodoform test
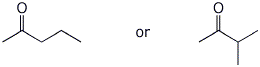
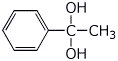
d) the hydrate of acetophenone
e) (3S,4R)-3,4-epoxy-1-pentanol
![]()
3. (15 points) Arrange the following in order with respect to the property indicated. Write MOST and LEAST under the compounds with the highest and lowest values, respectively.
a) basicity
![]()
b) reactivity with cyanide ion
![]()
c) ease of dehydration
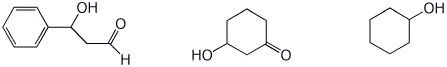
d) rate of reduction with NaBH4
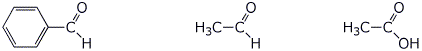
e) amount of enol at equilibrium
4. (15 points) Complete each of the following reactions by adding the missing part: either the necessary reagents and conditions or the structure of the expected major product. Show stereochemistry if it is specific.
a) 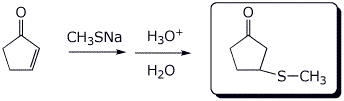
b) 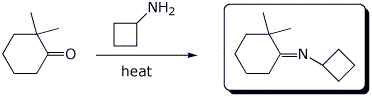
c) 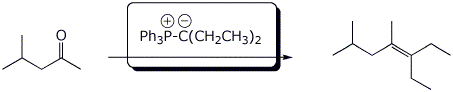
d), e) 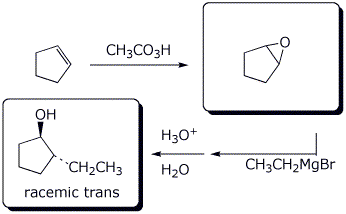
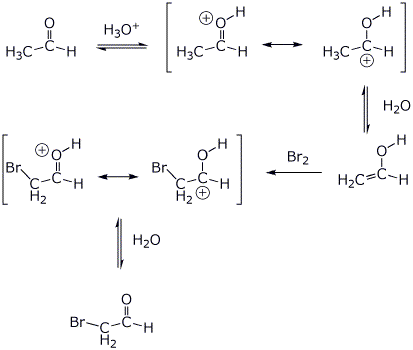
5. (10 points) Write a complete mechanism for the reaction below. Show all steps and all resonance forms for any intermediates involved.
![]()
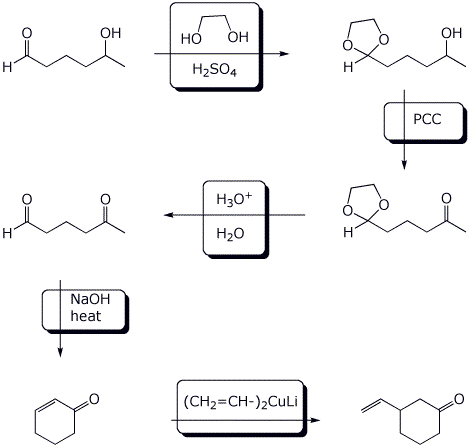
6. (15 points) Complete the following synthetic sequence by adding the appropriate reagents and conditions at each step.
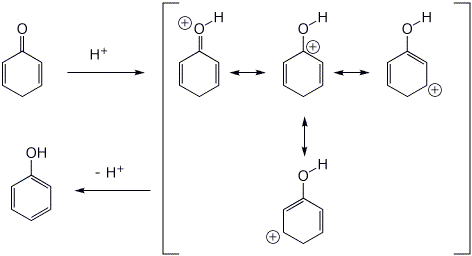
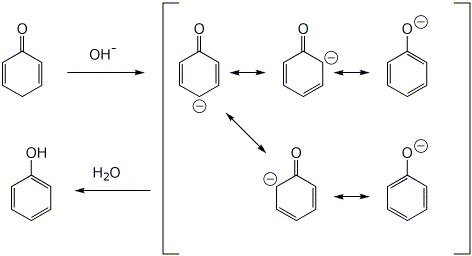
7. (15 points) Cyclohexadienones are unstable, readily converting to phenols with either acid or base catalysis. Write complete mechanisms for each case, showing all resonance forms for the intermediates involved.