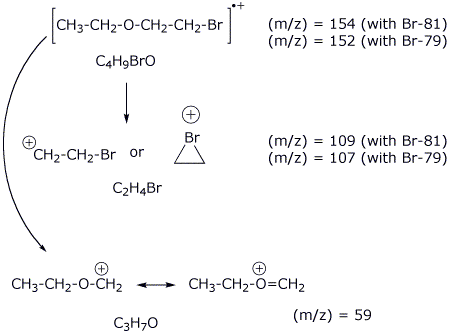Organic Chemistry II |
 |
|
Professor Carl C. Wamser |
||
Chem 335 - Winter 2008 |
Exam 2 Answer Key |
![]()
Organic Chemistry II |
 |
|
Professor Carl C. Wamser |
||
Chem 335 - Winter 2008 |
Exam 2 Answer Key |
![]()
1. (15 points) Write clear structures for each of the following:
a) (Z),(R)-4-hexen-2-ol
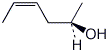
b) a chromate ester
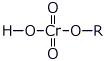
c) an isobutyl Gilman reagent
![]()
d) the periodic acid cleavage products from 1,2-propanediol
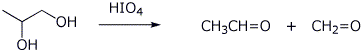
e) sodium 4-nitrobenzenethiolate
![]()
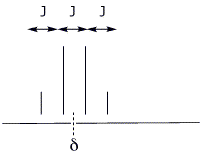
2a. (5 points) Draw a typical quartet from a proton NMR spectrum and show where the chemical shift (d) and the coupling constant (J) would be measured.
2b. (5 points) Give a specific example of the 18-electron rule, explaining how your example fits the rule.
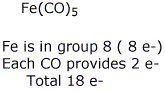
2c. (5 points) The formation of a vicinal diol
using tBuOOH and OsO4 is a syn addition.
Illustrate what this means in terms of the stereochemistry of the product
expected from cyclopentene. Draw the product(s) expected and designate
stereochemistry with R and S.
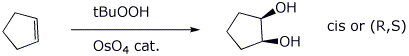
2d. (5 points) The formation of a vicinal
diol using tBuOOH and OsO4 is a syn addition.
Illustrate what this means in terms of the stereochemistry of the product
expected from
cis-2-butene. Draw the product(s) expected and designate stereochemistry
with R and S.

3. (15 points) Complete each of the following reactions by writing the structure of the expected product:
a) 
b) 
c) 
d) 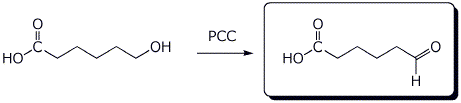
e) 
4. (20 points) Show how to prepare each of the compounds below using Grignard coupling reactions for the C-C bond-forming steps. All the carbons in the products must originate from alcohols having four or fewer carbon atoms. You may use any needed reagents or solvents.
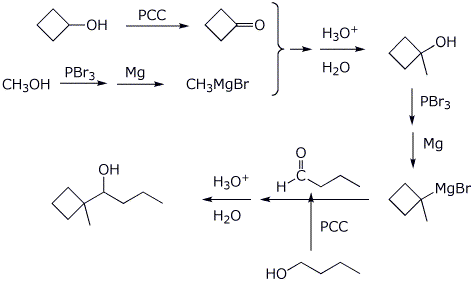
![]()

5. (15 points) Identify the unknown compound based on the data below.
molecular formula C9 H10 O2
What is the calculated IHD? 5
1H NMR :
a 9.9
ppm , 1 H , singlet
b 7.8
ppm , 2 H , doublet
c 6.9
ppm , 2 H , doublet
d 4.1
ppm , 2 H , quartet
e 1.4
ppm , 3 H , triplet
Identify by letter on your final structure each of the H absorptions above.
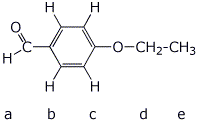
How many different peaks would be expected for this compound in its 13C NMR spectrum?
7
6. (15 points) Identify the unknown compound based on the data below. It has IHD = 0.
Mass spectrum: molecular ion at 152 & 154 (approximately
equal intensity peaks)
additional
peaks at 107 & 109 (equal intensity)
largest
peak at 59
13C NMR: peaks at 70, 66, 30, and 15 ppm
1H NMR :
a 3.7
ppm , 2 H , triplet
b 3.5
ppm , 2 H , quartet
c 3.4
ppm , 2 H , triplet
d 1.2
ppm , 3 H , triplet
Identify by letter on your final structure each of the H absorptions above.
![]()
Identify each of the 13C peaks by reference to your structure.
![]()
Identify each of the peaks in the mass spectrum.