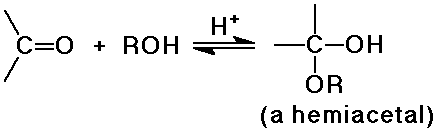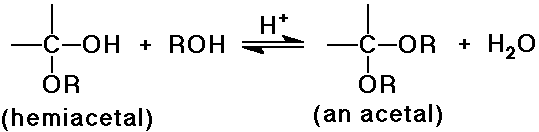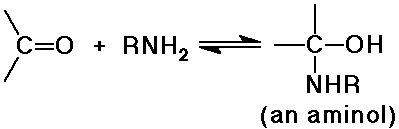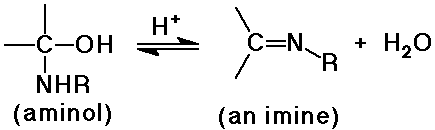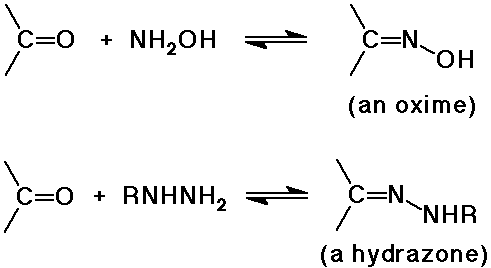Organic Chemistry II |
 |
|
Professor Carl C. Wamser |
||
Chem 335 - Winter 2008 |
Chapter 17 Notes |
![]()
Carbonyl Compounds
Organic Chemistry II |
 |
|
Professor Carl C. Wamser |
||
Chem 335 - Winter 2008 |
Chapter 17 Notes |
![]()
Carbonyl Compounds
![]()
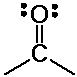
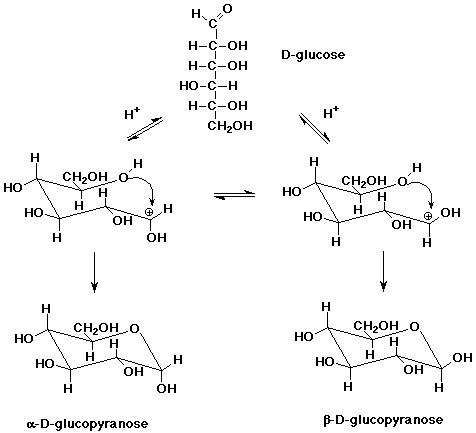
usually subdivided into two families:
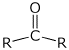
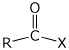
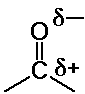
formaldehyde
acetaldehyde
propionaldehyde
butyraldehyde
benzaldehyde
acyl- as prefix
e.g., acetyl or benzoyl, as in acetylcyclopropane
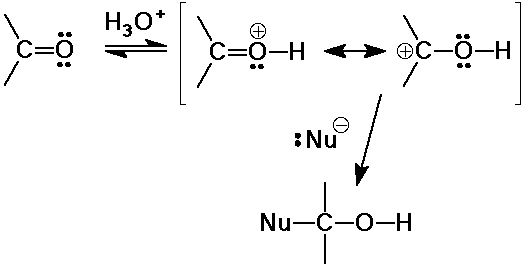
the major reaction of carbonyl compounds
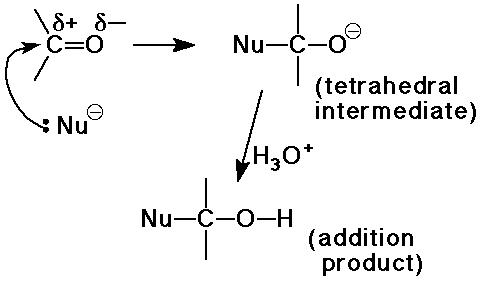
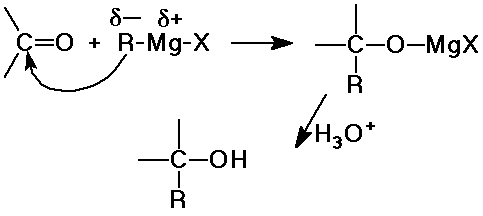
C nucleophiles: CN-, RLi, RMgX, ylids
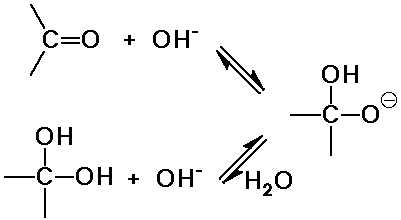
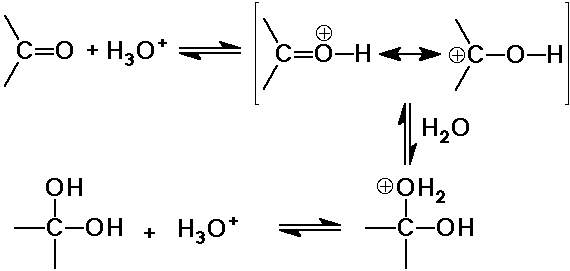
O nucleophiles: OH-, H2O, ROH
S nucleophiles: RSH
N nucleophiles: NH3, RNH2 , NH2OH, RNHNH2
H nucleophiles: LiAlH4, NaBH4
![]()