Organic Chemistry II |
 |
|
Professor Carl C. Wamser |
||
Chem 335 - Winter 2007 |
Quiz 11 |
![]()
Organic Chemistry II |
 |
|
Professor Carl C. Wamser |
||
Chem 335 - Winter 2007 |
Quiz 11 |
![]()
1. (2 points) Purine, shown below, is one of the bases in nucleic acids. Identify the type of atomic or hybrid orbital that would be expected for the lone pair on each of the four nitrogens.
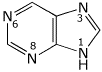
N1 - p orbital
N3, N6, N8 - sp2 orbitals
2. (2 points) Identify the number of cyclic pi electrons (electrons that would be counted in applying Hückel’s Rule) and whether each compound below would be classified as aromatic according to Hückel’s Rule.
12 pi electrons - not aromatic (antiaromatic, if planar)
6 pi electrons - aromatic
3. (6 points) Write all the resonance forms for the intermediate that would be expected in the substitution reaction shown below.
The para derivative (shown above) reacts faster than the meta derivative. Explain.
The benzylic cation in the para intermediate includes a resonance form at the carbon with the CH3 group, which is more stable.
The intermediate for meta would not have a resonance form at the carbon with the CH3 group.