Organic Chemistry II
Organic Chemistry II |
 |
|
Professor Carl C. Wamser |
||
Chem 335 - Winter 2007 |
Chapter 10 Homework Answers |
![]()
1. Write all the possible stereoisomers that could result from the Diels-Alder reaction of (E,E)-2,4-hexadiene with cis-2-butenedinitrile (cis-1,2-dicyanoethylene).
Given the preference for endo addition and minimizing steric hindrance, predict the major product(s). Would this product be described as racemic, achiral, or optically active?
First assume that we are only considering stereoisomers in which the stereochemistry of the diene and the alkene are retained, as it typically is in a Diels-Alder reaction. Without this assumption, there are many other possibilities.
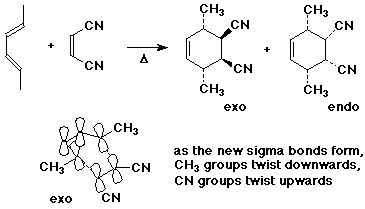
The endo product would be expected to be the major product based on electronic effects stabilizing the transition state (kinetic control). However, the exo product is more stable based on lesser steric repulsions between the substituents (thermodynamic control).
Since the reaction is not reversible, equilibration of the products is not expected and the kinetically controlled product (endo) is expected to be the major product.
Because of the symmetry of these molecules, both the exo and endo products are meso compounds (achiral). The realtionship of exo to endo is diastereomers.