Organic Chemistry II |
 |
|
Professor Carl C. Wamser |
||
Chem 335 - Winter 2007 |
Exam 3 |
![]()
Organic Chemistry II |
 |
|
Professor Carl C. Wamser |
||
Chem 335 - Winter 2007 |
Exam 3 |
![]()
1. (15 points) Write accurate names for the following:
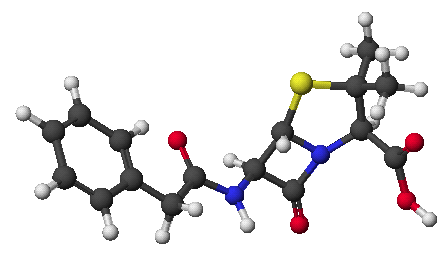
a) 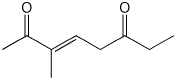
b) 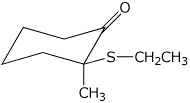
c) 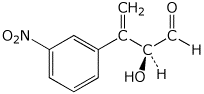
2. (15 points) Write accurate structures for the following:
a) cyclopentanone hydrazone
b) the ethyl hemiacetal of benzophenone
c) (R)-2,3-epoxy-2-methylpentane
d) the enolate anion of acetophenone (best resonance form)
e) diphenyl sulfone
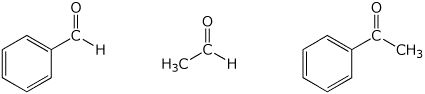
3. (15 points) Arrange the following in order with
respect to the property indicated.
Write MOST and LEAST under the compounds
with the highest
and lowest values, respectively.
a) amount of hydrate at equilibrium
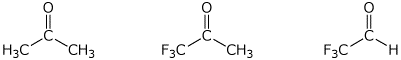
b) amount of (S) product expected

c) rate of bromine substitution in aqueous base
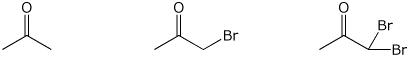
d) relative stability
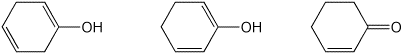
e) relative reactivity towards a typical nucleophile
4. (15 points) Complete each of the following reactions by writing the structure of the expected major product:
a) 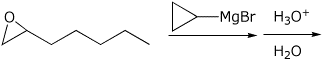
b) 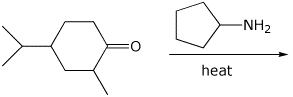
c) 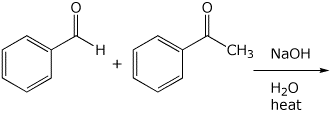
d) 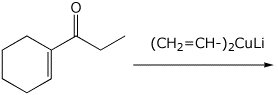
e) 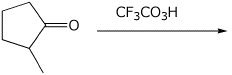
5. (15 points) Write a complete mechanism for the hydrolysis of the acetal
shown below.
Show all steps, all resonance forms for intermediates, and all electron-pushing
arrows.
Do NOT use H+ or -H+ ; show the proper acid-base reactions using the water
solvent.
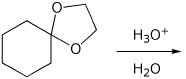
6. (15 points) Aldol condensations are reversible. Write a mechanism for
the isomerization shown below.
Show all steps and all resonance forms for
intermediates.
(Hint - 2,6-heptanedione is involved in the mechanism)
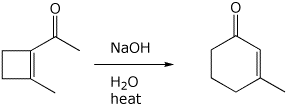
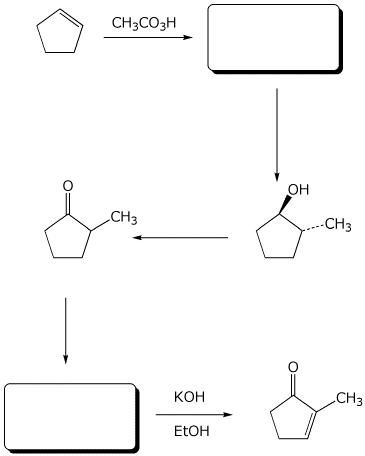
7. (10 points) Complete the following synthesis by adding the missing parts: