Organic Chemistry II |
 |
|
Professor Carl C. Wamser |
||
Chem 335 - Winter 2007 |
Exam 2 |
![]()
Organic Chemistry II |
 |
|
Professor Carl C. Wamser |
||
Chem 335 - Winter 2007 |
Exam 2 |
![]()
1. (15 points) Write accurate structures for the following:
a) lithium dibenzylcuprate
b) a nitrate ester
c) dibromocarbene (show all electrons around C, assuming sp2 hybridization)
d) ferrocene
e) starting from the position of Fe in the periodic table, explain why ferrocene is considered to follow the 18-electron rule
2. (15 points) Complete each of the following reactions by adding the missing part: either the structure of the expected major product or the necessary reagents and conditions:
a) 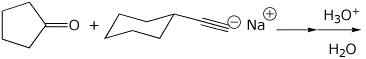
b) 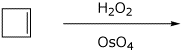
c) 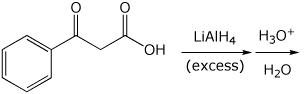
d) 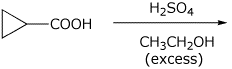
e) 
3. (10 points) Balance the following equation.
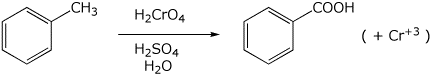
4. (10 points) Show electron-pushing arrows that convert the compounds shown into the products shown.
a) 
b) 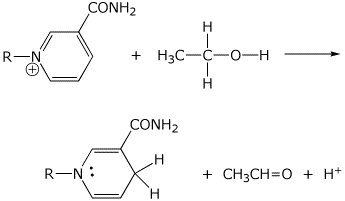
5. (20 points) Show synthetic sequences for each of the following compounds:
a) All carbons in the final product must originate from simple alcohols (no other functional groups) having four or fewer carbons.
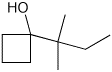
b) The carbons in the final product must originate from benzene, methyl iodide, and acetone (dimethyl ketone or 2-propanone are alternate names for acetone).
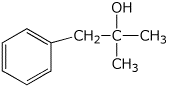
6. (15 points) Write the structure of the unknown compound that gives the data shown.
Molecular formula: C12H18
Calculate IHD =
The molecular ion in the mass spectrum would be expected at m/z =
H-1 NMR:
a 7.1 ppm, 4 H, singlet
b 2.3 ppm, 2 H, septet
c 1.3 ppm, 12 H, doublet
For each H-1 NMR peak indicated, show which H’s correlate with each peak.
C-13 NMR shows just four peaks, two in the aromatic region and two
in the aliphatic region.
Explain why this is consistent with your structure.
7. (15 points) Write the structure of the unknown compound that gives the data shown.
Molecular formula: C8H18O2
Calculate IHD =
The infrared spectrum does NOT show any peaks in the
range 3200-3600 cm-1
nor in the range 1600-1800 cm-1. Indicate what you conclude from
these data.
H-1 NMR:
a 5.2 ppm, 1 H, quartet
b 3.3 ppm, 2 H, quartet
c 2.3 ppm, 3 H, doublet
d 1.7 ppm, 3 H, triplet
e 1.5 ppm, 9 H, singlet
For each H-1 NMR peak indicated, show which H’s correlate with each peak.