Organic Chemistry II |
 |
|
Professor Carl C. Wamser |
||
Chem 335 - Winter 2007 |
Exam 1 Answer Key |
![]()
Organic Chemistry II |
 |
|
Professor Carl C. Wamser |
||
Chem 335 - Winter 2007 |
Exam 1 Answer Key |
![]()
1. (6 points) Write accurate structures for the following:
a) 3-nitro-4-phenylphenol
b) the 1,4-addition product of HBr and 1,3-butadiene
2. (9 points) Write ALL the resonance forms for
a) the benzyl radical
b) the intermediate in the Friedel-Crafts methylation of bromobenzene (just do para)
3. (10 points) For 1,3,5-hexatriene :
a) Write an energy level diagram, indicating the number of pi molecular orbitals expected, their relative energies, the number of electrons expected in each orbital, and identifying the HOMO and LUMO.
b) Draw approximate molecular orbital pictures of the HOMO and LUMO of 1,3,5-hexatriene, indicating orbital symmetries and the presence of any nodal planes.
4. (10 points) A particular substituent X is identified as having the following partial rate factors for electrophilic aromatic bromination:
ortho - prf = 12 , meta - prf = 3, para - prf = 30
Substituent X would be classified as ACTIVATING or DEACTIVATING (circle one)
The relative rate of reaction compared to benzene would be ___ 10 x faster than benzene ___
( 2 x 12 ) + ( 2 x 3 ) + 30 = 60 (compared to benzene = 6)
The directing effect of substituent X would be described as ___ ortho,para-directing ___
The product mixture after bromination of X-benzene would be expected to be
____40____% ortho ( 24/60 )
____10____% meta ( 6/60 )
____50____% para ( 30/60 )
5. (15 points) Arrange the following in order with respect to the property indicated. Write MOST and LEAST under the compounds with the highest and lowest values, respectively.
a) stability
1,2-pentadiene / / / trans-1,3-pentadiene / / / 1,4-pentadiene
LEAST / / / MOST / / / MIDDLE
b) reactivity in an SN1 reaction
LEAST / / / MIDDLE / / / MOST
c) reactivity in a Diels-Alder reaction
MOST / / / LEAST / / / MIDDLE
d) reactivity in a nitration reaction
MOST / / / MIDDLE / / / LEAST
e) reactivity towards bromine
LEAST / / / MOST / / / MIDDLE
6. (15 points) Complete each of the following reactions by writing the structure of the expected major product:
a) 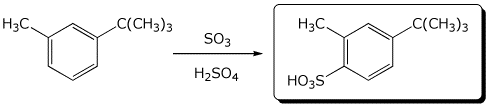
b) 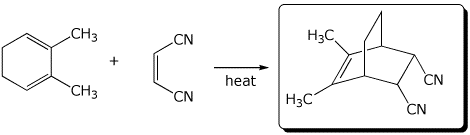
c) 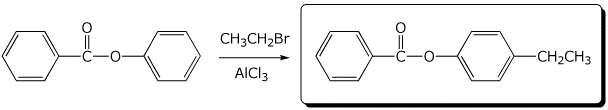
d) 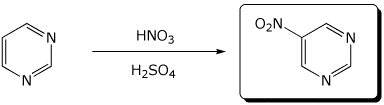
e) 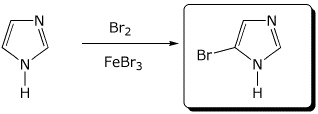
7. (15 points) The four compounds listed below undergo SN1 reactions at increasingly faster rates. For each compound, write all the resonance forms for the cation intermediate that would be formed.
Explain why the resonance forms provide information about the rate differences.
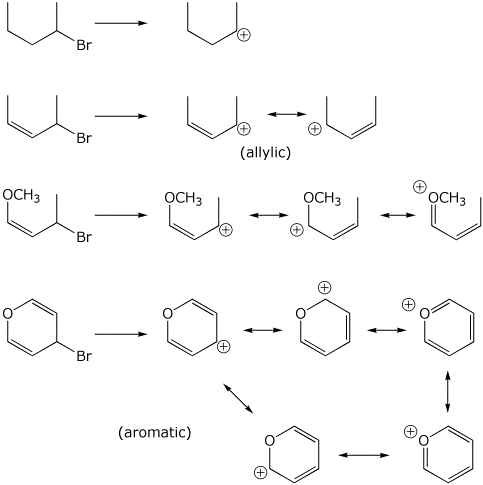
In general, the more resonance forms and the better the resonance forms, the more stable the cation will be. The resonance forms above include allylic forms, delocalization to an O lone pair, and aromaticity.
When the cation intermediate is more stable, the activation energy to form it is likely to be lower, leading to a faster rate.
8. (10 points) Complete the following reaction sequence by filling in the boxes with the missing structures or reagents and conditions.
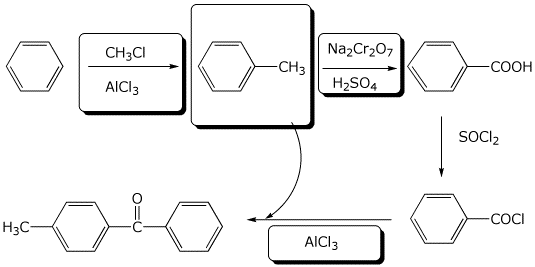
9. (10 points) Starting from benzene, show a sequence of reactions that could be used to synthesize meta-chlorobenzoic acid.