Organic Chemistry II |
 |
|
Professor Carl C. Wamser |
||
Chem 335 - Winter 2006 |
Quiz 10 |
![]()
Organic Chemistry II |
 |
|
Professor Carl C. Wamser |
||
Chem 335 - Winter 2006 |
Quiz 10 |
![]()
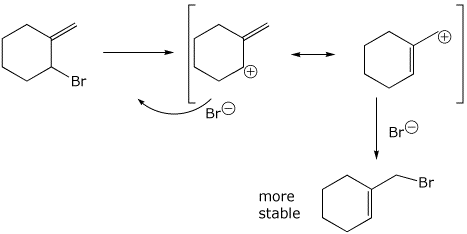
1. (2 points) The compound below undergoes an allylic rearrangement when heated in a polar aprotic (non-nucleophilic) solvent; it gives a more stable isomer of the original compound. Write a mechanism that shows the intermediate carbocation with its resonance forms and the final product.
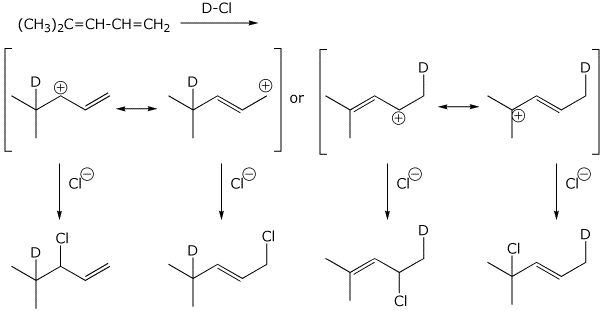
2. (6 points) Addition of DCl to the diene shown below gives four possible products, based on addition at either end and the possibilities of 1,2 or 1,4 addition. Write out a complete mechanism to show all four products and the cation intermediates involved, with resonance forms. Just add one equivalent of DCl.
3. (2 points) Cyclopentadiene is such a good Diels-Alder diene that it reacts with itself. Show the major product expected.
