Organic Chemistry II |
 |
|
Professor Carl C. Wamser |
||
Chem 335 - Winter 2006 |
Final Exam |
![]()
Organic Chemistry II |
 |
|
Professor Carl C. Wamser |
||
Chem 335 - Winter 2006 |
Final Exam |
![]()
1. (25 points) Write complete names for each of the following.
a) 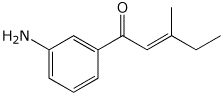
b) 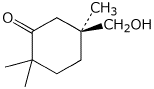
c) 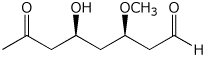
d) 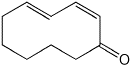
e) 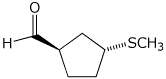
2. (15 points) Write accurate structures for the following:
a) benzyl sec-butyl ether
b) the conjugate acid of pyridine
c) the best resonance form for the anion ![]()
d) the HOMO of cyclopentadiene
e) an aromatic anion with five carbons
3. (20 points) Identify the unknown compounds based on the information provided.
a) Compound A: C5H10 has only one peak in a C-13 NMR
b) Compound B: C5H12 has only one peak in a H-1 NMR
c) Compound C: C6H8O an aldol condensation product from hexanedial
d) Compound D: C8H10 can be oxidized by hot chromic acid to benzoic acid
e) Compound E: C10H12 a Diels-Alder dimer of cyclopentadiene
4. (15 points) Complete the following reactions by adding the missing part: either the necessary reagents and conditions or the final major product.
a) 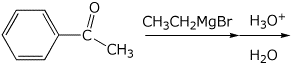
b) 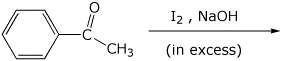
c) 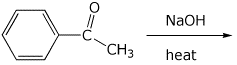
d) 
e) 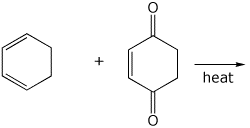
5. (15 points) Complete the following reactions by adding the missing part: either the necessary reagents and conditions or the final major product.
a) 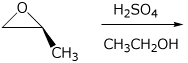
b) 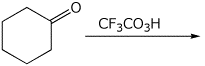
c) 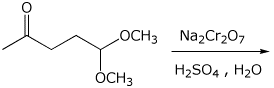
d) 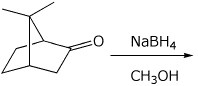
e) ![]()
6. (15 points) Each of the following compounds are easily hydrolyzed in
dilute aqueous acid.
Indicate the products that would be formed in each case.
a) 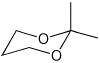
b) 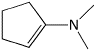
c) 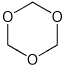
d) ![]()
e) 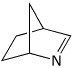
7. (20 points) Write a complete mechanism for the formation of an acetal in the reaction shown below. Show all steps and show all resonance forms for any intermediates involved.
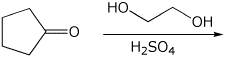
8. (15 points) Write a complete mechanism for the aldol condensation (with dehydration) shown below. Show all steps and show all resonance forms for any intermediates involved.
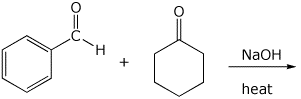
9. (20 points) Write a complete mechanism for the substitution reaction
shown below.
Show all steps and show all resonance forms for any intermediates involved.
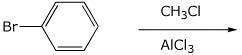
10. (20 points) Show how to synthesize the compounds below.
a) starting materials are limited to benzene and alcohols having three carbons or fewer
* include a Grignard synthesis
b) using cyclohexane and methanol as the only source of carbons in the product
* include an epoxide
11. (20 points) Identify the unknown compound that has all the following characteristics.
a) The molecular formula is C11H16O2 . Calculate the index of hydrogen deficiency.
b) The IR spectrum shows no absorptions in the
range 3100-3500 or 1700-1800 cm-1.
What can you conclude from these data?
c) The proton NMR shows the following four absorptions. Correlate the hydrogens in your final structure with each peak.
A 7.5 ppm, 5H, multiplet
B 3.8 ppm, 6H, singlet
C 1.8 ppm, 2H, quartet
D 1.1 ppm, 3H, triplet
d) The mass spectrum shows a strong peak at m/z = 149 , as well as the parent molecular ion at m/z = 180. Explain what structure the 149 peak corresponds to.
e) How many different C-13 peaks would be expected for this compound.