Organic Chemistry II |
 |
|
Professor Carl C. Wamser |
||
Chem 335 - Winter 2006 |
Exam 3 Answer Key |
![]()
Organic Chemistry II |
 |
|
Professor Carl C. Wamser |
||
Chem 335 - Winter 2006 |
Exam 3 Answer Key |
![]()
1. (15 points) Write complete names for each of the following.
a) 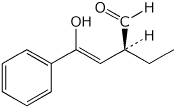
(S,Z)-2-ethyl-4-hydroxy-4-phenyl-3-butenal
b) 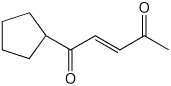
(E)-1-cyclopentyl-2-penten-1,4-dione
c) 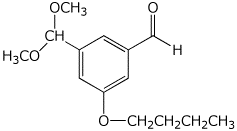
3-butoxy-5-(dimethoxymethyl)benzaldehyde
2. (15 points) Write accurate structures for the following:
a) the diethyl acetal of cyclobutanecarbaldehyde
b) cis-1-methoxy-1,2-epoxybutane
c) the enolate of (E)-3-hexenal (best resonance form)
d) dibenzyl sulfoxide
e) the oxime of acetophenone
3. (15 points) Write all the resonance forms for the initial intermediate that would be formed in the following reactions.
acid-base reactions:
a) 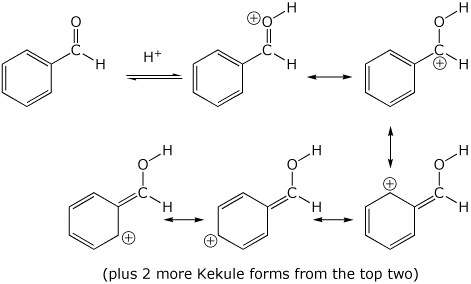
b) 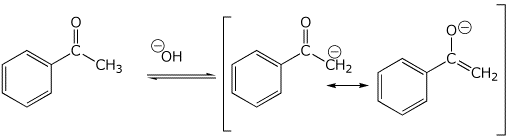
c) 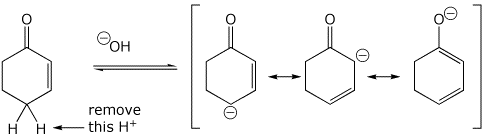
nucleophilic addition reactions:
d) 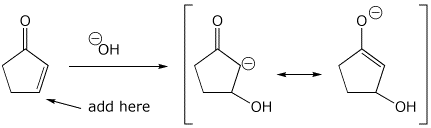
e) 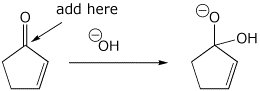
4. (15 points) Complete each of the following reactions by adding the missing part: either the starting compound, the necessary reagents and conditions, or the final major product.
a) 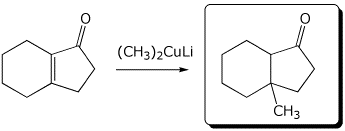
b) 
c) 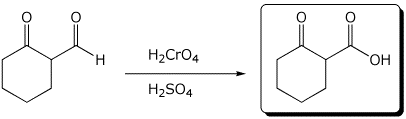
d) 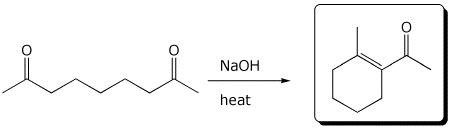
e) 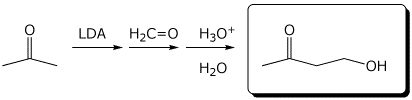
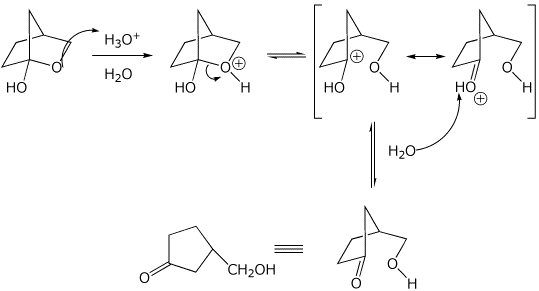
5. (15 points) Write a complete mechanism for the acid-catalyzed hydrolysis of the hemiacetal shown below. Show all steps and all resonance forms.
(Hint - the mechanism is the exact reverse of the acid-catalyzed formation of a hemiacetal)
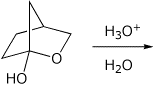
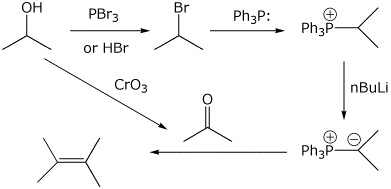
6. (10 points) Show how to synthesize 2,3-dimethyl-2-butene, beginning with 2-propanol as the only carbon source and using a Wittig reaction.
7. (15 points) Write a complete mechanism for the acid-catalyzed formation of the enamine shown below. Show all steps and all resonance forms.
