Organic Chemistry II |
 |
|
Professor Carl C. Wamser |
||
Chem 335 - Winter 2006 |
Exam 1 |
![]()
Organic Chemistry II |
 |
|
Professor Carl C. Wamser |
||
Chem 335 - Winter 2006 |
Exam 1 |
![]()
1. (15 points) Write accurate structures for each of the following.
a) 2-allyl-4-nitrophenol
b) trans-4-benzylcyclohexanol
c) the HOMO of 1,3-cyclopentadiene
d) cycloheptatrienyl cation
e) s-cis-4-methyl-1,3-pentadiene
2. (15 points) Write ALL the resonance forms for the intermediate that would be formed in each reaction. Final products are not required.
a) 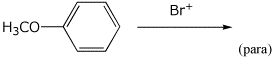
b) 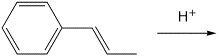
c) 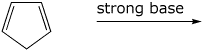
3. (15 points) Complete each of the following reactions by adding the missing part: either the starting compound, the necessary reagents and conditions, or the final major product. Include stereochemistry of it is specific.
a) 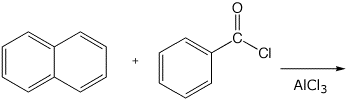
b) 
c) 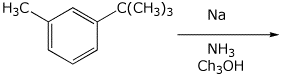
d) 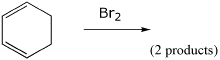
e) 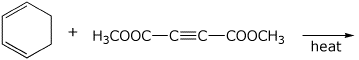
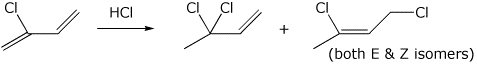
4. (15 points) The reactions shown below indicates two products from addition of HCl to chloroprene. Identify each as a 1,2 or 1,4 product. Write a complete mechanism, including all resonance forms for any intermediates.
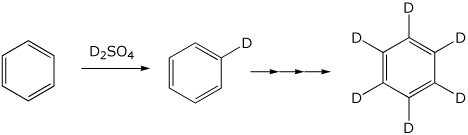
5a. (10 points) Benzene can be fully deuterated by treatment with D2SO4 . Write a complete mechanism (including all resonance forms for any intermediates) for the substitution of H by D as in the first reaction shown below (do just one substitution).
5b. (10 points) Tert-butyl groups can be removed from an aromatic ring by treatment with strong acid like H2SO4 . Write a complete mechanism, including all resonance forms for any intermediates.
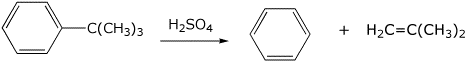
6. (20 points) Show a sequence of reactions that could be used to prepare each of the following compounds. The original aromatic compound must be benzene.