Organic Chemistry II |
 |
|
Professor Carl C. Wamser |
||
Chem 335 - Winter 2005 |
Chapter 16 Workshop |
![]()
Organic Chemistry II |
 |
|
Professor Carl C. Wamser |
||
Chem 335 - Winter 2005 |
Chapter 16 Workshop |
![]()
![]()
Purpose: In this workshop you will explore ring-opening reactions of epoxides. You should be able to identify oxidation and reduction reactions and predict the stereochemistry and regiochemistry of additions to epoxides.
Expectations: You should review and be prepared for
an initial discussion about the meaning of the following key words and concepts:
a) oxidation-reduction, oxidation state
b) stereochemistry of addition
c) regiochemistry of addition
Problems :
1. Identify the oxidation state of each carbon in the following functional groups. For each pairwise transformation, describe it as an oxidation, a reduction, or neither (there are 12 combinations to consider). For each transformation, indicate a reagent or sequence of reactions that would be appropriate to accomplish the transformation.
2. Each of the following synthetic reactions failed. Explain what went wrong and offer alternate synthetic approaches.
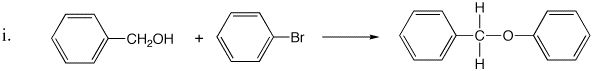
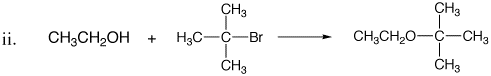
3. Propose a reasonable mechanism for the reaction below, in which compound A is transformed to compound B by reaction with base. Use the arrow formalism to show the flow of electrons.
4. In order to determine the regiochemistry of epoxide hydration, various epoxides have been studied in aqueous solutions enriched with O-18. Results of addition of H2O-18 to different epoxides are summarized below.
R |
R' |
catalyst |
% a-substitution |
% b-substitution |
CH3 |
H |
acid |
70 |
30 |
CH3 |
H |
base |
20 |
80 |
CH3 |
CH3 |
acid |
100 |
0 |
CH3 |
CH3 |
base |
0 |
100 |
C6H5 |
H |
acid |
100 |
0 |
C6H5 |
H |
base |
0 |
100 |
Explain these results with separate acid-catalyzed
and base-catalyzed mechanisms.
Why does the propylene oxide give mixed results?
In a later report, researchers found that the base-catalyzed reaction with styrene oxide actually gives about an equal mix (50:50) of a- and b-substitution. To support their case, they studied optically active styrene oxide and found that the base-catalyzed mechanism gives racemic product. Why does this support their case? How does the styrene oxide case compare with the mixed results seen in the propylene oxide case?
Reflection: Consider the experimental issues involved in determining a reaction mechanism. Epoxide additions involve both regiochemical and stereochemical issues, with possible stereochemistry at each carbon. How do you expect the location of O-18 was detected? How is the absolute stereochemistry of a stereogenic center verified? Can you think of an experiment that would track the stereochemistry at the unsubstituted (1°) carbon?
Materials adapted from:
Peer-Led Team Learning: Organic Chemistry, 1/e
Jack A. Kampmeier, University of Rochester
Pratibha Varma-Nelson, St. Xavier University
Donald Wedegaertner, University of the Pacific
Prentice-Hall, 2001, ISBN 0-13-028413-0
http://www.sci.ccny.cuny.edu/~chemwksp/OrganicChem.html