Organic Chemistry II |
 |
|
Professor Carl C. Wamser |
||
Chem 335 - Winter 2005 |
Final Exam |
![]()
Organic Chemistry II |
 |
|
Professor Carl C. Wamser |
||
Chem 335 - Winter 2005 |
Final Exam |
![]()
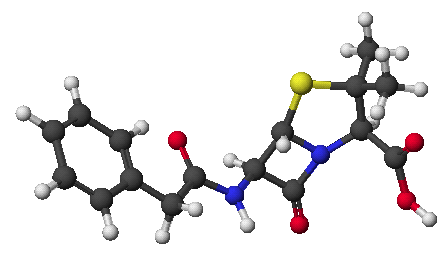
1. (25 points) Write complete names for each of the following, including stereochemistry if it is specifically shown.
a) 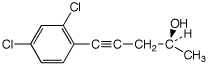
b) ![]()
c) ![]()
d) 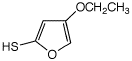
e) 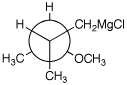
2. (15 points) Write accurate structures for each of the following.
a) o-isopropyl benzyllithium
b) 3-methylthiopyridine
c) ferrocene
d) dimethyl sulfoxide
e) the HOMO of s-cis-1,3-butadiene
3. (15 points) Compare each of the following with respect to the property indicated. Write “MOST” and “LEAST” under the compounds with the highest and lowest values of the property indicated.
a) boiling point
b) ease of oxidation
c) reactivity towards nitration
d) activation of a benzene ring towards bromination
e) relative stability (MOST means most stable = lowest energy)
4. (15 points) Complete each of the following reactions by adding the missing part: either the starting compound, the necessary reagents and conditions, or the expected major product. Show stereochemistry if it is specific.
a) ![]()
b) ![]()
c) 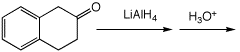
d) 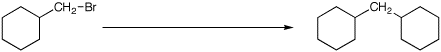
e) ![]()
5. (15 points) Complete each of the following reactions by adding the missing part: either the starting compound, the necessary reagents and conditions, or the expected major product. Show stereochemistry if it is specific.
a) 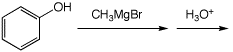
b) 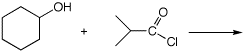
c) 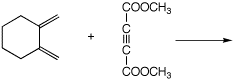
d) 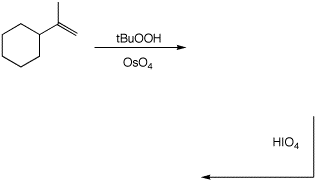
e)
6. (20 points) Write a complete mechanism for the nitration of anisole to form para-nitroanisole, shown below. Show all steps, including the formation of the active electrophile from nitric acid. Show all resonance forms for the intermediates involved.
7. (20 points) The compound below exists in two tautomeric forms, readily
interconverted by either acid or base.
Write all the resonance forms for
the intermediate formed under each of those two conditions.
In acid:
In base:
8. (20 points) Show a sequence of reactions that could be used to synthesize each of the two the compounds shown below. Show all steps and the product of each step. You may start with benzene, acetylene, ethylene oxide, and any alcohol having four or fewer carbons as your source of carbon for the final product.
9. (15 points) Write a complete mechanism for the addition of one equivalent
of HBr to 4-methyl-1,3-pentadiene. Select the initial addition so as
to form the more stable intermediate and write both resonance forms.
Predict the kinetic
and thermodynamic products.
10. (20 points) Identify the unknown compounds based on the information
given below.
Calculate IHD for each compound, and for the NMR spectra, identify the
hydrogens or carbons that give rise to each absorption described.
a) C4 H10 O
no IR absorptions in the range 3000 - 3500 cm-1 or 1500 - 2000 cm-1
1-H NMR: 3.7 ppm, 1H septet ; 3.4 ppm, 3H singlet ; 1.9 ppm, 6H, doublet
IHD =
b) C10 H14
13-C NMR shows only four absorptions: 20, 40, 120, 150 ppm
1-H NMR: 1.0 ppm, 6H triplet ; 2.4 ppm, 4H quartet ; 7.0 ppm, 4H, singlet
IHD =
11. (20 points) Write a complete mechanism for the reaction shown below. Show all steps and electron-pushing arrows.
Describe the expected 1-H NMR spectrum of the final product.
Correlate
hydrogens with individual peaks using notation as on the previous page.