Organic Chemistry II |
 |
|
Professor Carl C. Wamser |
||
Chem 335 - Winter 2005 |
Exam 2 Answer Key |
![]()
Organic Chemistry II |
 |
|
Professor Carl C. Wamser |
||
Chem 335 - Winter 2005 |
Exam 2 Answer Key |
![]()
1. (15 points) Write complete names for each of the following, including stereochemistry if it is specifically shown.
a) 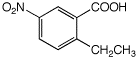
2-ethyl-5-nitrobenzoic acid
b) 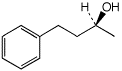
(S)-4-phenyl-2-butanol
c) 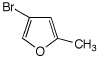
4-bromo-2-methylfuran
d) 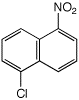
1-chloro-5-nitronaphthalene
e) ![]()
p-isobutylaniline
4-(2-methylpropyl)benzenamine
2. (15 points) Write accurate structures for each of the following:
a) p-nitrobenzoic acid
b) 2,6-dimethylpyridine
c) 2-(4-methoxyphenyl)ethanol
d) the HOMO of benzene (either one)
e) the product of a Birch reduction on o-diethylbenzene
3. (15 points) Compare each of the following with respect to the property indicated. Write “MOST” and “LEAST” under the compounds with the highest and lowest values.
a) reactivity towards Br2
![]()
LEAST / / MOST / / MIDDLE
b) amount of para substitution expected
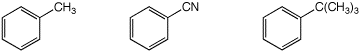
MIDDLE / / LEAST / / MOST
c) SN1 reactivity
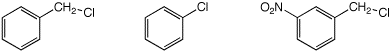
MOST / / LEAST / / MIDDLE
d) reactivity in a Friedel-Crafts alkylation
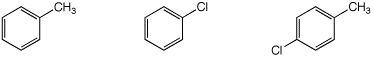
MOST / / LEAST / / MIDDLE
e) relative energy level (MOST = most stable, or lowest energy)
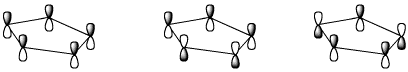
MOST / / MIDDLE / / LEAST
4. (15 points) Complete each of the following reactions by adding the missing part: either the starting compound, the necessary reagents and conditions, or the expected major product.
a) 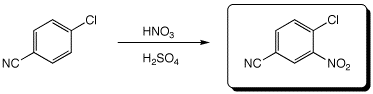
b) 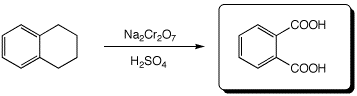
c) 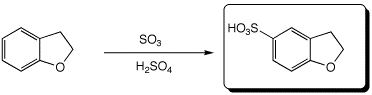
d) 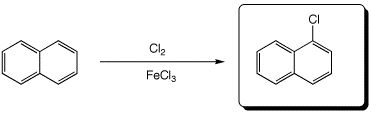
e) 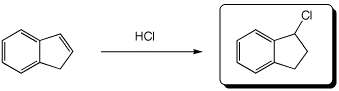
5. (20 points) Write a synthetic sequence of reactions that could be used to prepare the following compounds, starting from benzene.
6. (10 points) Write a complete mechanism for the bromination of chlorobenzene
to form
p-bromochlorobenzene. Show all steps and show all resonance forms for any
intermediates involved.
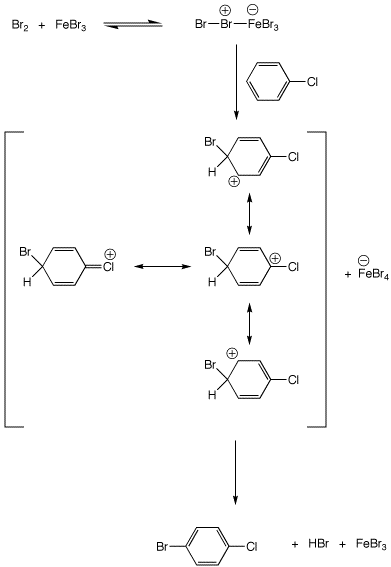
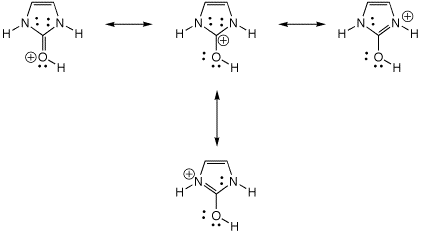
7. (10 points) The heterocycle shown below has
two tautomeric forms, which are readily interconverted by acid.
Identify which structures (A, B, and/or C) would be classified as aromatic.
Write all the resonance forms for B, including lone pairs.
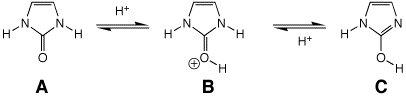
B and C are aromatic.