|
|
Organic Chemistry
II
|
|
Professor Carl C. Wamser
|
|
|
Chapter 12 Notes
|

Electrophilic Aromatic Substitution

Electrophilic Aromatic Substitution
- benzene can be made to react with very strong electrophiles (E+)
- intermediate is a carbocation
(like addition to one of the pi bonds)
- nucleophiles don't add to the cation
(H+ leaves, regenerates benzene ring)
- reaction is substitution (E+ for H+)
Mechanism of Aromatic Substitution
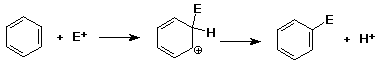
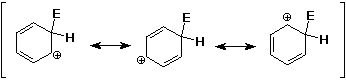
Mechanism - why slower than alkenes ?
- Ea for electrophilic attack on benzene is greater than Ea for electrophilic
attack on an alkene
- although the cation intermediate is delocalized and more stable than
an alkyl cation, benzene is much more stable than an alkene
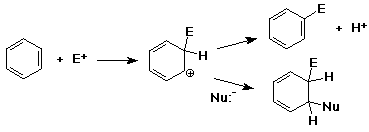
Mechanism - why substitution ?
- the substitution product regains the aromatic stability
- an addition product would be a conjugated diene, not as stable
Bromination of Benzene
- electrophile is Br+
- generated from Br2 + FeBr3
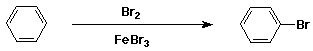
Chlorination of Benzene
- electrophile is Cl+
- generated from Cl2 + FeCl3
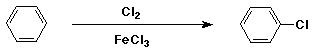
Nitration of Benzene
- electrophile is NO2+
- generated from H2SO4 + HNO3
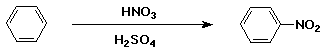
Sulfonation of Benzene
- electrophile is HSO3+
- generated from H2SO4 + SO3
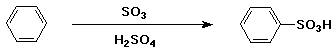
Friedel-Crafts Alkylation
- electrophile is an alkyl cation (R+)
- generated from RCl + AlCl3
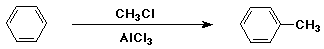
- limitations of the Friedel-Crafts reaction
- cation rearrangements may occur
- doesn't work with deactivated aromatic rings
- extensions of the Friedel-Crafts reaction
- other sources of cations, e.g., alkene + H+
Friedel-Crafts Acylation
- electrophile is an acyl cation (RCO+)
- generated from RCOCl + AlCl3
- acyl chlorides prepared from RCOOH + SOCl2
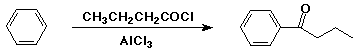
- Clemmensen reduction: Zn(Hg) / HCl - reduces C=O to CH2
- Wolff-Kishner reduction: NH2NH2 / KOH / 180° - reduces C=O to CH2
- acylation followed by reduction makes primary alkylbenzenes without
rearrangement
Substituent Effects
- substituents on the benzene ring can affect the reaction in two ways:
reactivity - substituted benzene may react faster or slower than
benzene itself reacts
orientation - the new group may be oriented ortho, meta, or para
with respect to the original substituent
Reactivity Effects
- activating - reaction is faster
observed with electron-donating groups that make the ring more electron-rich
- deactivating - reaction is slower
observed with electron-withdrawing groups that make the ring less electron-rich
Orientation Effects
- substituent already present on the benzene ring determines the location
of the new group
- ortho,para-directors: electron-donating groups direct the new
group mainly to ortho & para
- meta-directors: electron-withdrawing groups direct new group
mainly meta
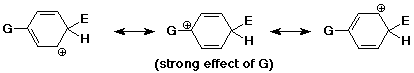
Ortho, Para Directors
- the best cation is formed when the electrophile adds either ortho or
para
(better than unsubstituted)
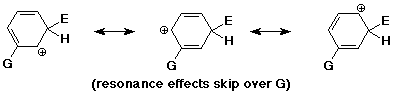
Meta Directors
- the best cation is formed when the electrophile adds meta
(but this is worse than unsubstituted)
Classifying Substituents
- see Table 12.2
- activating and o,p-directing:
alkyl, aryl, O and N groups
- deactivating and m-directing:
N+ groups, polar multiple bonds
- deactivating but o,p-directing:
the halogens (F, Cl, Br, I)
(electron-withdrawing atoms, but lone pairs can stabilize the cation when
it is ortho or para)
Partial Rate Factors
- rate of reaction at one position relative to benzene
- activating and o,p-directing:
CH3 : o= 42 , m = 2.5 , p = 58
- deactivating and m-directing:
CF3 : o = 0.0000045 , m = 0.000067 , p = 0.0000045
- deactivating but o,p-directing:
Cl : o = 0.029 , m = 0.0009 , p = 0.137
Synthetic Strategy
- synthesis of complex compounds requires attention to the order in which
groups are attached
- retrosynthetic analysis - think backwards one step at a time
(What reaction could have made this target compound?)
Synthesis Example
- target compound: p-nitrobenzoic acid
- note both substituents are m-directors
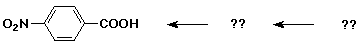
Synthesis Example
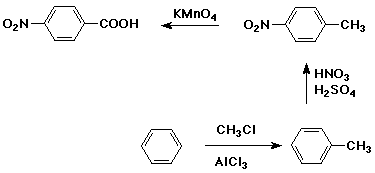
Substitution on Other Aromatic Systems
- naphthalene - 1-position (activated)
- furan, pyrrole, thiophene - 2-position (activated)
- pyridine - 3-position (deactivated)
![]()

![]()
![]()



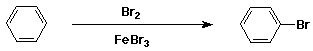
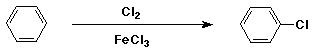
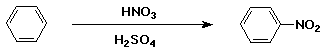
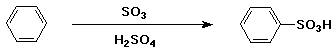
![]()
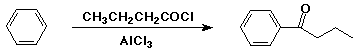


![]()
