Organic Chemistry II |
 |
|
Professor Carl C. Wamser |
||
Chem 335 - Winter 2004 |
Chapter 13 Quiz |
![]()
Organic Chemistry II |
 |
|
Professor Carl C. Wamser |
||
Chem 335 - Winter 2004 |
Chapter 13 Quiz |
![]()
![]()
1. (4 points) Propose structures for the following based on the proton NMR data given:
a) C5H11Cl
singlet, 9H, 1.0 ppm and singlet, 2H,
3.3 ppm
neopentyl chloride (CH3)3CCH2Cl
b) C4H8Br2
singlet, 6H, 1.8 ppm and singlet, 2H, 3.8 ppm
1,2-dibromo-2-methylpropane (CH3)2CBrCH2Br
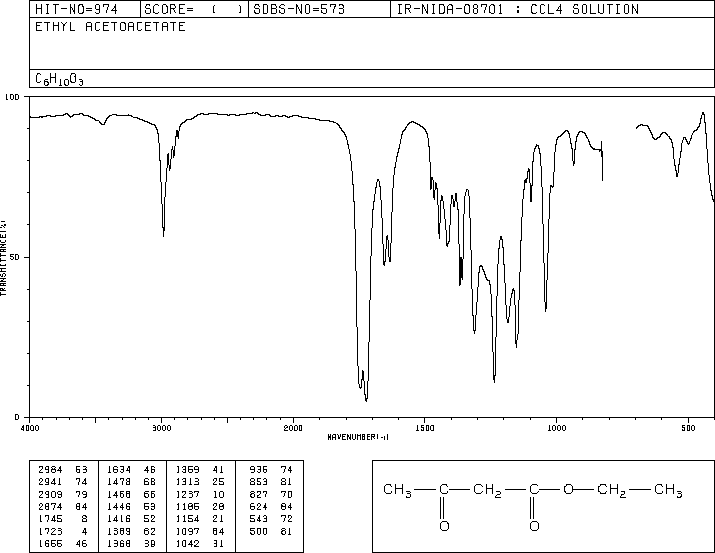
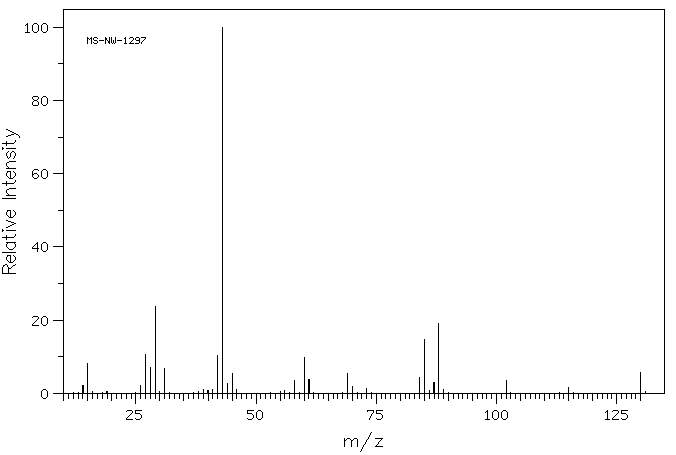
2. (6 points) Identify the unknown compound that gives the spectra shown
on the attachment.
Assign all peaks in the proton and C-13 NMR spectra
(correlate letters on the spectra with carbons and hydrogens in the structure).
Identify at least one point of information obtained from:
a) index of hydrogen deficiency
C6H10O3 indicates IHD = 2 (sum of rings + pi bonds)
b) mass spectrum
parent molecular ion at 130 indicates molecular weight
c) infrared spectrum
strong peaks around 1600 cm-1 indicate C=O (possibly two types)
ethyl acetoacetate CH3COCH2COOCH2CH3
IR Spectrum:
Mass Spectrum:
H-NMR Spectrum:

C-NMR Spectrum:
