Organic Chemistry II |
 |
|
Professor Carl C. Wamser |
||
Chem 335 - Winter 2004 |
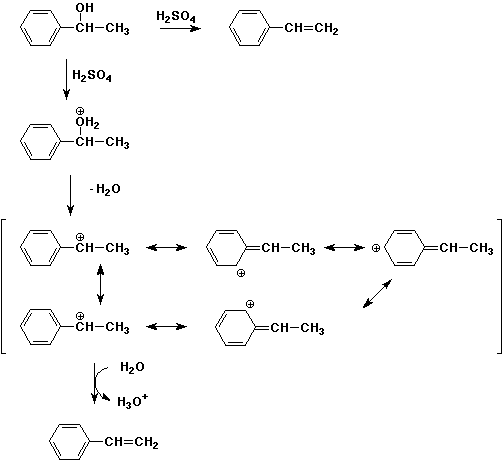
Chapter 11 Quiz |
![]()
Organic Chemistry II |
 |
|
Professor Carl C. Wamser |
||
Chem 335 - Winter 2004 |
Chapter 11 Quiz |
![]()
![]()
1. (5 points) Write the five major resonance forms for the aromatic hydrocarbon phenanthrene, shown below without its seven pi bonds.
see above