Organic Chemistry II |
 |
|
Professor Carl C. Wamser |
||
Chem 335 - Winter 2004 |
Chapter 12 Homework Answers |
![]()
Organic Chemistry II |
 |
|
Professor Carl C. Wamser |
||
Chem 335 - Winter 2004 |
Chapter 12 Homework Answers |
![]()
Carey, pages 512 - 518 :
Problems 12.22 - 45
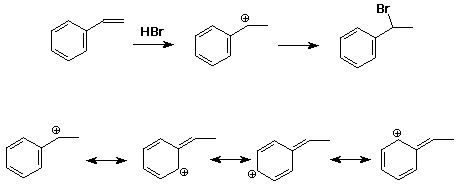
1. Write a complete mechanism for the Markovnikov addition of HBr to styrene. Show all resonance forms for the intermediate.
2. Consider whether the following substituents would be activating or deactivating, and also predict whether they would be meta- or ortho,para-directing. Write the best Lewis structures for each substituent to justify your answers.
-CNO , -NCO , -OCN
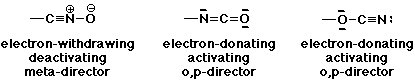
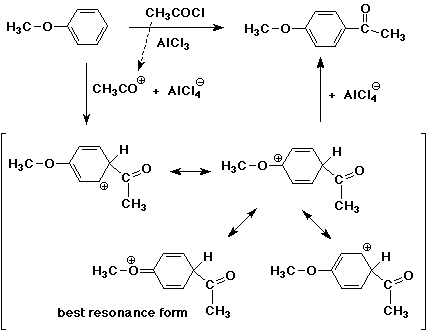
3. Write a complete mechanism for the Friedel-Crafts reaction of anisole with acetyl chloride, including all resonance forms for the intermediate. Indicate the most stable resonance form.