Organic Chemistry II |
 |
|
Professor Carl C. Wamser |
||
Chem 335 - Winter 2004 |
Exam 3 |
![]()
Organic Chemistry II |
 |
|
Professor Carl C. Wamser |
||
Chem 335 - Winter 2004 |
Exam 3 |
![]()
1. (15 points) Write accurate structures for each of the following, including stereochemistry if it is specifically indicated.
a) 12-crown-4
b) dimethyl sulfoxide
c) isobutyl phenyl ether
d) a p-methoxybenzyl Grignard reagent
e) dichlorocarbene
2. (15 points) Complete each of the following reactions by adding the missing part: either the starting compound, the necessary reagents and conditions, or the final major product. Show stereochemistry if it is specific.
a) 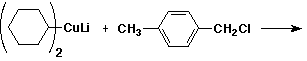
b) 
c) ![]()
d) ![]()
e) 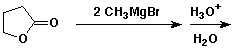
3. (15 points) Complete each of the following reactions by adding the missing part: either the starting compound, the necessary reagents and conditions, or the final major product. Show stereochemistry if it is specific.
a) 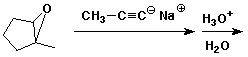
b) 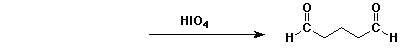
c) 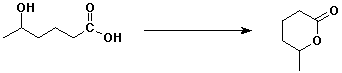
d) 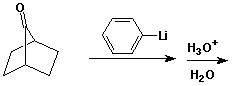
e) 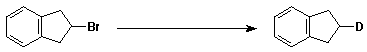
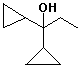
4. (20 points) Write synthetic sequences that could be used to prepare the following compounds.
a) you may begin with any alcohols having three carbons or fewer
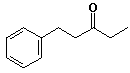
b) you may begin with benzene, ethylene oxide and any alcohols having three carbons or fewer
5. (15 points) Write two complete mechanisms that illustrate the reaction of (R)-styrene oxide (shown below) with methanol under either basic or acidic conditions.
Pay particular attention to both the regiochemistry and stereochemistry in each mechanism.
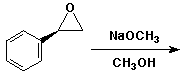
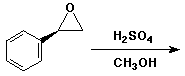
6. a. (10 points) Write a complete mechanism for the reaction shown below.
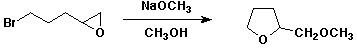
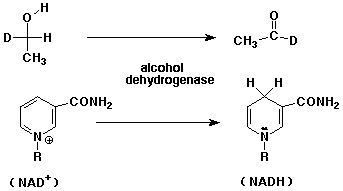
b. (10 points) The oxidation of ethanol by NAD+ catalyzed by alcohol dehydrogenase
is stereospecific. This was determined by experiments such as the one shown
below (i.e., only H and not D is removed).
What is the stereochemistry of the deuterated ethanol shown?
Show the electron-pushing arrows that illustrate how deuterated ethanol (the specific isomer shown) is oxidized by NAD+.