Organic Chemistry II |
 |
|
Professor Carl C. Wamser |
||
Chem 335 - Winter 2004 |
Exam 1 Answers |
![]()
Organic Chemistry II |
 |
|
Professor Carl C. Wamser |
||
Chem 335 - Winter 2004 |
Exam 1 Answers |
![]()
1. (15 points) Write complete names for each of the following, including stereochemistry if it is specifically shown.
a) 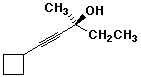
(S)-1-cyclobutyl-3-methylpent-1-yn-3-ol
b) ![]()
(4E,7Z)-8-bromonona-4,7-dien-1-yne
c) ![]()
sodium 1-pentynide
2. (15 points) Complete each of the following reactions by adding the missing part: either the starting compound, the necessary reagents and conditions, or the final major product. Show stereochemistry if it is specific.
a) 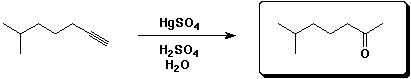
b) 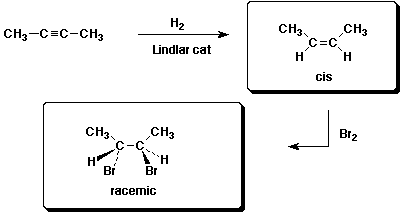
c)
d) 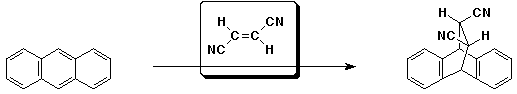
e) 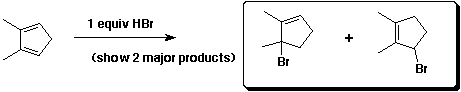
3. (15 points) Write a complete mechanism for the addition of one equivalent of Cl2 to the diene shown below, including all possible 1,2 and 1,4 products. Show all steps and show all resonance forms for any intermediates involved.
4. (20 points) Write clear structures for all the stereoisomers that would be formed in the following Diels-Alder reaction. Label the products with letters (A, B, C, etc.). For every possible pair, identify which are enantiomers, which are diastereomers, and which are constitutional or any other type of isomers. Predict which are the preferred products.
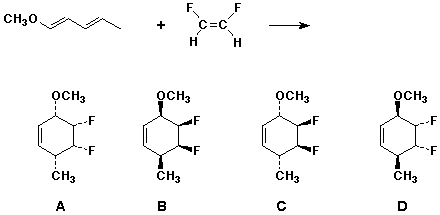
A and B are the endo (preferred) products.
A/B and C/D are enantiomer pairs.
All other pairs are diastereomers ( A/C , A/D , B/C , B/D ).
5. (15 points) Show a sequence of reactions that would be used to synthesize 3,4-dichlorohexane, starting from acetylene as the ONLY source of the carbons in your final product. The stereochemistry of the product is not a concern.
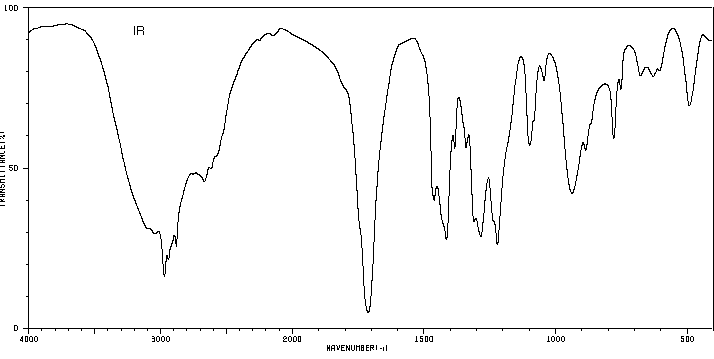
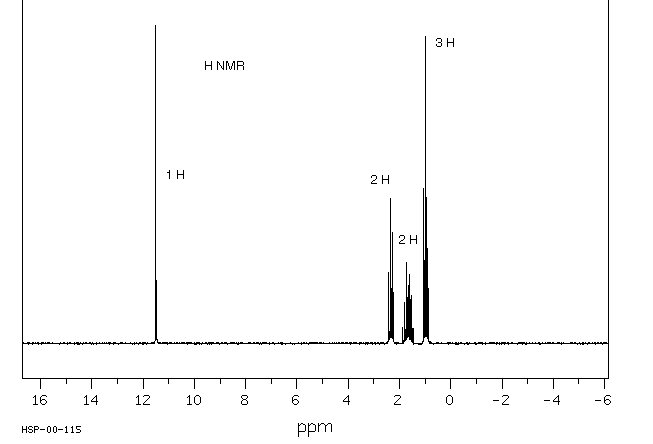
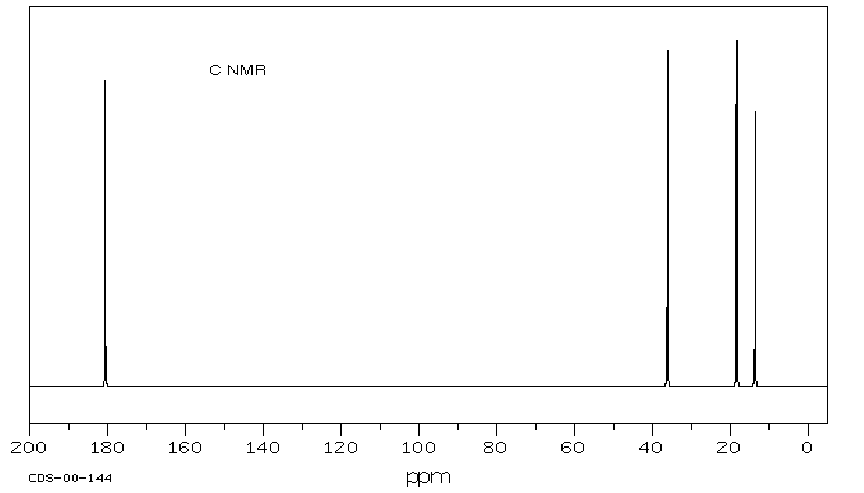
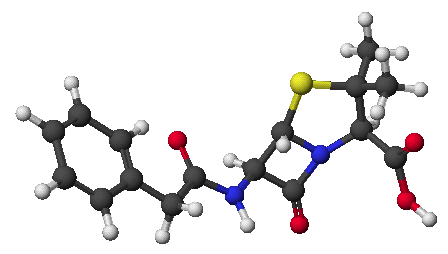
6. (20 points) Identify compound A, which gives the spectra
on the following page.
Also identify compound B, which is formed upon ozonolysis of compound
A and gives the spectra on the subsequent page.
Identify at least two structural features in each IR spectrum, and identify
all the peaks in each of the proton and carbon NMR spectra.
Compound A:
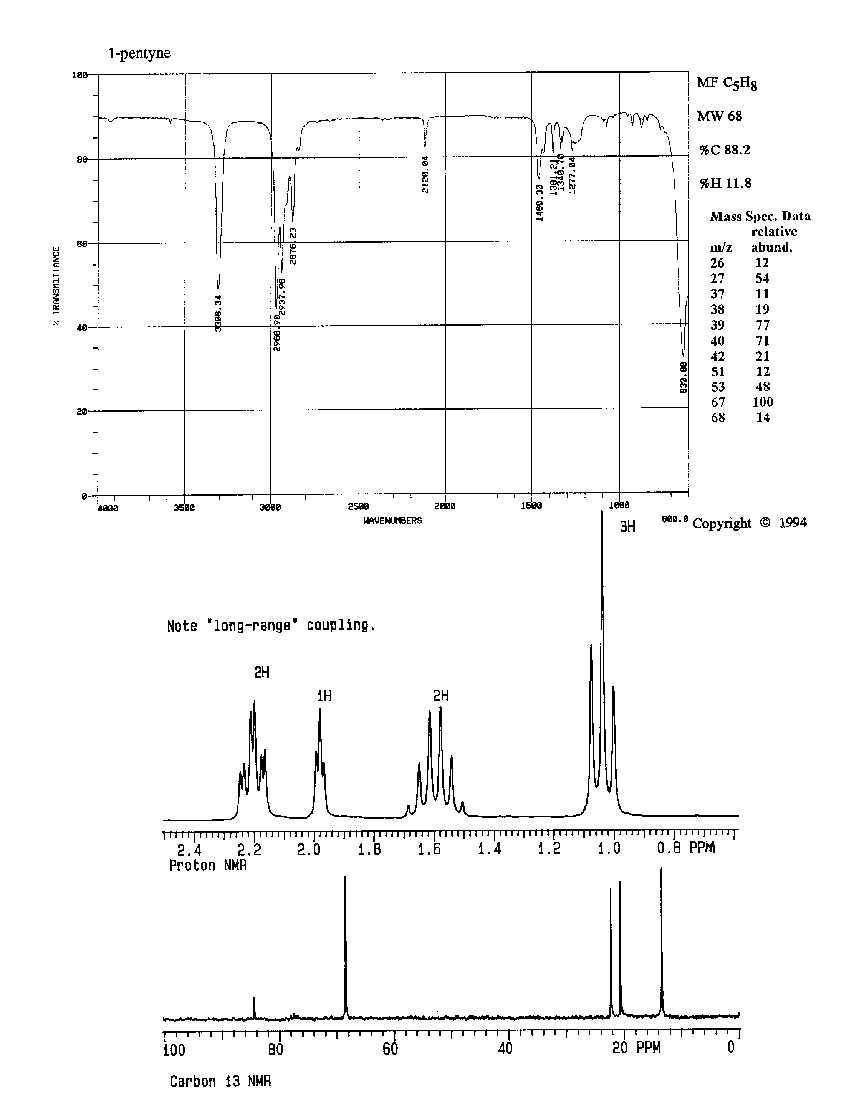
Compound B: