|
|
Organic Chemistry
II
|
|
Professor Carl C. Wamser
|
|
|
Chapter 13 Notes
|

Spectroscopy / Structure Determination

Determination of the Structure of Organic Compounds
- a variety of methods apply, mainly using specialized instruments
- spectroscopy - absorption of light of various wavelengths
- "wet" methods - inferences from specific chemical
reactivity
- comparisons with known samples or databases
Properties of Light
- as electromagnetic waves:
c = l v ( lambda * nu )
c = speed of light (3 x 10^8 m/sec)
l = wavelength
v = frequency (per sec)
- as particles (photons):
E = h v
h = Planck's constant
The Electromagnetic Spectrum
- x-rays ~ 0.1 - 10 nm
- ultraviolet ~ 10 - 400 nm
- visible ~400 - 800 nm
- infrared ~ 1 - 100 µm
- microwaves ~ 0.01 - 10 cm
- radiowaves ~ 0.1 - 100 m
(0.1 - 100 MHz - compare AM/FM frequencies)
Absorption Spectroscopy
- spectrum: plot of absorbance as a function of wavelength
(or frequency)
- y-axis: absorbance or transmittance
- x-axis: wavelength range
- peaks of absorbance (or valleys of transmission)
represent specific molecular interactions with the light
Molecular Energy Levels
- energy levels are quantized
- absorption occurs when the energy of the light (per photon)
exactly matches the energy difference between an occupied and
an unoccupied energy level
- E = E2 - E1 = h v
- absorption spectroscopy reveals the spacings between energy
levels in a molecule
Types of Molecular Energy
- nuclear > 500 kcal/mol - gamma rays
- electronic ~ 100 kcal/mol - ultraviolet/visible
- vibrational ~ 1 kcal/mol - infrared
- rotational ~ 1 cal/mol - microwaves
- magnetic* ~ 0.1 cal/mol - radiowaves
* induced by a strong external magnetic field
NMR for nuclear magnetic effects
EPR for electron magnetic effects
Nuclear Spin States
- many nuclei have a magnetic moment (nuclear spin)
- H nucleus (proton) has two possible spin quantum states (up
& down)
- C-13 also has two states
- other magnetic nuclei include F-19, P-31, H-2, N-14
- different states are distinguishable with an external magnetic
field
Nuclear Magnetic Resonance
- an applied magnetic field makes the two spin states unequal
in energy
- a photon can be absorbed (resonance), when
- the precession frequency matches the photon frequency
- which happens when the photon energy matches the energy difference
- thereby promoting a nucleus with the lower energy spin
to the higher energy spin state
- typical magnetic fields used are large, but cause small energy
splitting
- spin populations are very close to 50:50, but resonance can
still occur
NMR Instrumentation
- larger magnetic fields require higher frequency radiowaves
(30, 60, 90, 200, 400, 500 MHz instruments are typical)
- for 21,000 Gauss,
H-1 nuclei resonate at about 90 MHz
C-13 nuclei resonate at about 22.6 MHz
- Note - although C-13 is only about 1.1% of all carbon in
nature, this is sufficient for most samples to show good spectra
MRI - Magnetic Resonance Imaging
- special technique for spatial visualization of H nuclei in
a whole body
- distinct forms of H2O and organic
H are visualized
Equivalent Hydrogens
- equivalent hydrogens absorb together
- e.g., only one absorption for methane, ethane, ethylene,
benzene
- but two for propane, butane, isobutane
- diastereotopic hydrogens - replacement would give diastereomers
(e.g., CH2 of propene)
- enantiotopic hydrogens - replacement would give enantiomers
(e.g., CH2 of chloroethane)
- enantiotopic hydrogens are magnetically equivalent (absorb
together - one peak)
Chemical Shifts
- different environments for individual hydrogens
cause them to absorb at slightly different resonance positions
- shielded/upfield:
higher electron density requires a stronger field for resonance
- deshielded/downfield:
lower electron density requires a weaker field for resonance
(using a constant radiofrequency)
TMS as a Reference
- the protons (and carbons) of (CH3)4Si are highly shielded
- TMS is set as the reference (zero on the chemical shift delta
scale)
- delta (ppm units) = shift from TMS in Hz / radiofrequency
in MHz
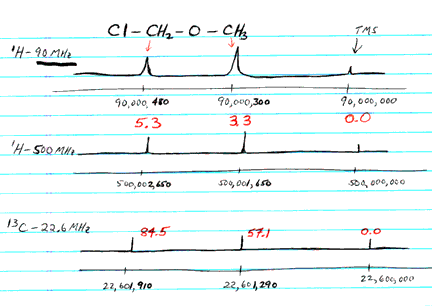
- e.g., Cl-CH2-O-CH3
shows 2 absorptions for H NMR and two for C NMR
- on a 90 MHz NMR, H resonances are at 90,000,300 Hz and at
90,000,480 Hz
(the instrument is tuned so that TMS absorbs at exactly 90,000,000
Hz)
- delta = 300 Hz / 90 MHz = 3.3 ppm
- delta = 480 Hz / 90 MHz = 5.3 ppm
- for C NMR, the absorptions are at 22,601,290 Hz and 22,601,910
Hz
(where TMS is at 22,600,000 Hz)
- delta = 1290 Hz / 22.6 MHz = 57.1 ppm
- delta = 1910 Hz / 22.6 MHz = 84.5 ppm
- Note that on a higher-field instrument (e.g., 500 MHz),
the chemical shifts would be the same
although the absolute differences (in Hz) would be larger
e.g., the proton resonances would be at
500,001,650 Hz (3.3 ppm) and
500,002,650 Hz (5.3 ppm)
- higher fields afford higher resolution:
- better separation of absorptions
- resonances typically look sharper at higher fields
Integrated Peak Areas
- indicate relative abundance of each type of hydrogen (or
carbon)
- experimentally usually shown as stair-steps
with the relative heights indicating relative area
- e.g., methyl tert-butyl ether shows two H NMR absorptions,
with integrated areas in the ratio 1:3,
representing the 3 methyl Hs and the 9 tert-butyl Hs
- the C NMR shows 3 peaks in relative areas 1:1:3
(however, instrumental methodology often makes integration of
C-13 peaks less reliable than H integrations)
- problem - C4H7Br3 gives only two H NMR peaks, in area ratio
4:3
Chemical Shift Correlations
- different functional groups lead to distinctive chemical
shifts
- generally, electron withdrawal leads to deshielding (higher
shifts)
- Table 13.1 will be provided for quizzes and exams
Spin-Spin Splitting Patterns
- recognize common splitting patterns
- singlet - a single sharp peak
- doublet - two equal peaks
- triplet - three peaks in the ratio 1:2:1
- quartet - four peaks in the ratio 1:3:3:1
The (n+1) Rule
- in general, when there are n neighboring Hs, the absorption
will be split to (n+1) lines
- e.g., CH3-CHCl2
two H NMR absorptions at 2.5 ppm and 5.9 ppm (area ratio
3:1)
- the peak at 2.5 ppm due to the methyl group is a doublet
(the chemical shift of 2.5 ppm is measured at the middle of the
doublet)
- the methyl Hs either feel a shielding or a deshielding effect
from their neighbor (C-H),
depending on whether its spin is aligned with or against the
applied magnetic field
- the neighboring C-H is half the time aligned with the magnetic
field
and half the time aligned against the magnetic field,
so the methyl peak is split to two equal peaks
- the CH peak is split to a quartet by its 3 neighboring methyl
Hs
- statistically, the 3 Hs can be aligned in four different
ways
(all with, all against, 2 with + 1 against, or 2 against + 1
with),
with the latter two possibilities more likely
Coupling Constant, J (Hz)
- the separation of the lines of a multiplet is J, the coupling
constant, measured in Hz
- all peaks in a multiplet are separated by the same amount,
J
- all peaks in the spin-coupled multiplet are separated by
the same amount, J
- coupling is strong for nearest neighbors H-C-C-H,
and small for more distant neighbors
- equivalent Hs don't split one another
(i.e., ethane is a singlet, not 2 quartets)
- the value of J (e.g., about 7 Hz for simple alkyl groups)
is the same regardless of the field strength of the instrument
- low-field instruments often give overlapping multiplets,
which is less of a problem in high-field instruments
C-13 NMR Spectra
- in principle, similar to H-1 NMR (two spin states)
- proton coupling indicates Hs on the same C
but spectra are often done with proton decoupling (simpler)
- chemical shifts :
- sp3 carbons 0 - 100 ppm
- sp2 carbons 100 - 200 ppm
- C=O carbons 170 - 210 ppm
- Table 13.3 will be provided for quizzes and exams
Interpretation of NMR Spectra - Solving Puzzles
- How many absorptions are there?
indicates number of equivalent kinds of Hs
- What are their intensities?
indicates number of Hs of each type
- What are their chemical shifts?
indicates the environment of each type of H
- What is the splitting pattern?
indicates neighboring Hs
- Create fragments with increasing detail
- Confirm interrelationships
- Correlate with any other information available
Infrared Spectroscopy
- probes different molecular vibrations
- absorption occurs when the frequency of the IR wave
matches a vibrational frequency of the molecule
- molecules typically have many possible vibrations
- bond stretching vibrations are most characteristic
(detect different kinds of A-B bonds)
- bond bending involves several atoms at once
more complex vibrations (rock, scissor, wag, twist)
- one exception: a completely symmetrical bond vibration does
not absorb IR
e.g., the C=C double bond stretch in ethylene is IR-silent
IR Spectra
- x-axis:
wavelength range typically 2 - 15 µm
wavenumber also used (second x-axis)
wavenumber = reciprocal wavelength (in cm-1)
- y-axis:
usually transmittance (0 - 100%)
(valleys correspond to absorptions)
"peak" position noted by wavenumber
Regions of an IR Spectrum
- Bonds to H (4000 - 2500 cm-1)
- Triple Bonds (2500 - 2000 cm-1)
- Double Bonds (2000 - 1500 cm-1)
- "Fingerprint" (1500 - 600 cm-1)
- Table 13.4 will be provided for quizzes and exams
Interpreting an Unknown IR Spectrum
- work from left to right (decreasing wavenumber)
- look for characteristic bond stretches
- try to confirm functional groups with additional absorptions
- if possible, compare to a known spectrum
Ultraviolet/Visible Spectroscopy
- x-axis:
typically 200 - 800 nm
- y-axis:
typically absorbance in % or log units
(peaks correspond to absorptions)
peak positions noted by lmax
Electronic Absorption Spectra
- uv/vis absorptions correspond to electron transitions
from one molecular orbital to another
- generally pi bonds are clearly seen in the uv region
- absorptions are called pi->pi* transitions
(from bonding to antibonding orbitals)
- one pi bond (ethylene) 165 nm (l max)
- conjugated dienes and polyenes absorb at longer wavelengths
(lower energies, or more closely spaced orbitals)
- 1,3-butadiene 217 nm
- benzene 254 nm
- beta-carotene 483 nm (absorbs violet - looks yellow)
- Table 13.5 will be provided for quizzes and exams
Photochemistry
- what do molecules do once an electron is promoted to a higher
energy orbital?
- many possibilities:
- emit light (fluorescence)
- lose energy as heat
- rearrange
- decompose
- transfer an electron to (or from) another molecule
Mass Spectrometry
- sample converted to a beam of ions ( usually +, sometimes
- )
- fragmentation of ionized molecules may take place
- fragments are collected by mass ( actually mass/charge ratio,
but charge is usually +1 )
- original structure is deduced from
- parent molecular ion (without fragmentation)
- fragments and their abundance
Instrumental Operation
- various ways of generating ions from a sample
- EI - electron ionization - high-energy beam of electrons
knock electrons off the sample molecules
- CI - chemical ionization - uses gas-phase reactions like
proton transfer
- FAB - fast atom bombardment - also knocks out electrons by
impact
- MALDI - matrix-assisted laser desorption ionization - laser
blasts ions out of a matrix (solid)
- magnetic field causes deflection of the ions as they travel
- bends them into a curve
- light ions (low m/z) are deflected most
- heavy ions are deflected less
- mass analyzer - the ions are spatially sorted by m/z and
detected as an ion current
Mass Spectrum
- a plot of ion current observed as the detector scans over
m/z values
- base peak - highest intensity peak (usually assigned intensity
100)
- parent molecular ion ( M+ or just M )
- corresponds to the original molecule missing one electron
- correlates with the molecular weight
- low-resolution MS - detects integer m/z values ( ±
1 )
- high-resolution MS - capable of identifying m/z to ±
0.0001
- e.g., CO and N2 both have MW = 28
but differ at high resolution
Isotopes
- some elements have distinctive isotope patterns in their
natural abundance
- C consists of 98.9% C-12 and 1.1 % C-13
- a compound with 20 C would give an M+1 peak about 22% the
size of M
- Br consists of about 50% each of Br-79 and Br-81 (atomic
weight ~ 80)
- a compound with one Br would have M and M+2 peaks about equal
in size
- Cl consists of about 75% Cl-35 and 25% Cl-37
- a compound with one Cl would have M and M+2 peaks in about
3:1 ratio
Fragmentation Patterns
- initial ionization generates a radical ion by loss of one
electron
- loss of a bonding electron may lead to bond breakings
- different functional groups often have characteristic fragmentation
patterns
Interpretation of Mass Spectra
- compounds containing only C, H, O, S, halogen, will always
have M even
- an even (m/z) corresponds to a radical ion
- an odd (m/z) corresponds to a carbocation
- the nitrogen rule - compounds with one N (or an odd number
of N) will have M odd
- a significant sized M+2 peak can indicate Br, Cl, or S (depending
on intensity)
- alkanes tend to fragment C-C bonds that give more
stable cations or radicals
- alkenes usually show a strong M+, fragment at allylic
bonds (forms allyl cations)
- alcohols fragment easily, losing H2O
or breaking an alpha C-C bond (forms oxonium ion)
- PRACTICE with spectra to learn how to visualize the information
they contain
![]()

![]()
![]()