
Chem 335 - Winter 2002
Organic Chemistry II
Dr. Carl C. Wamser

1. (15 points) Write complete names for each of the following:
a) 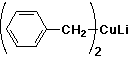
lithium dibenzyl cuprate
b) 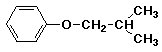
isobutyl phenyl ether
OR 2-methylpropoxybenzene
c) 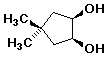
cis-4,4-dimethylcyclopentane-1,2-diol
d) 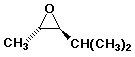
trans-2-methyl-3-(methylethyl)oxirane
OR trans-2-isopropyl-3-methyloxirane
OR trans-4-methyl-2,3-epoxypentane
e) ![]()
p-methoxyphenylmagnesium bromide
OR 4-methoxyphenylmagnesium bromide
2. (15 points) Write clear structures or illustrations for each of the following:
a) the disulfide obtained on oxidation of methanethiol
![]()
b) the sulfone obtained on oxidation of diphenyl sulfide
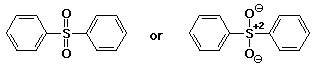
c) a sulfonate ester
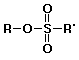
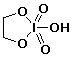
d) a cyclic periodate ester
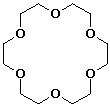
e) 18-crown-6
3. (15 points) Complete each reaction by adding the missing part: either the starting materials, the necessary reagents and conditions, or the final major product. Include stereochemistry if it is specific.
a) 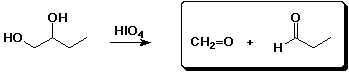
b) 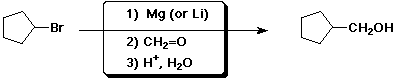
c) 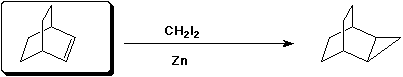
d) 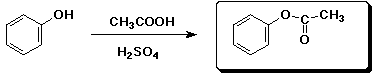
e) 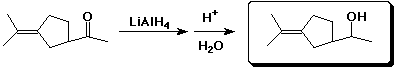
4. (15 points) Write a balanced equation for the following reaction:
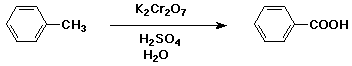
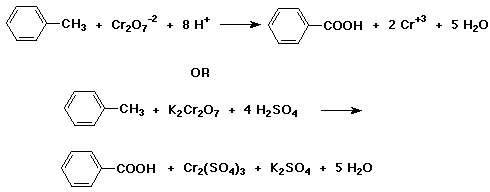
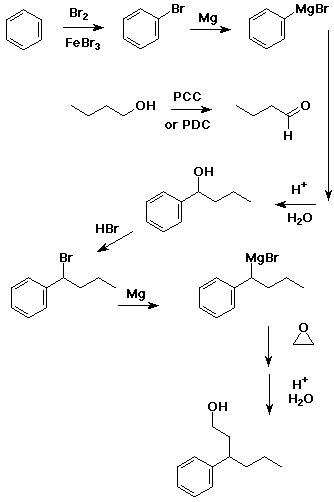
5. (15 points) Write a synthetic sequence of reactions that could be used to make the compound shown below. Your carbon sources must be benzene, 1-butanol, and ethylene oxide.
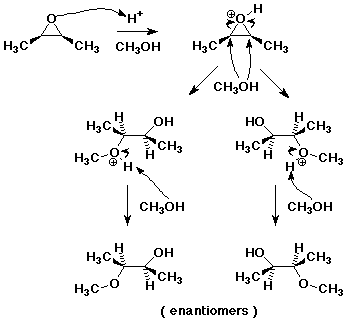
6. (15 points) Write a complete mechanism for the acid-catalyzed addition of methanol to cis-2,3-dimethyloxirane. Show all steps and show electron-pushing arrows and show clear stereochemistry to form ALL of the possible stereoisomeric products.
7. (10 points) The cyclic ether oxane can be formed from either of the two starting materials shown below. One requires acid and one requires base (you have to figure out which is which). Show complete mechanisms for each reaction.
![]()
![]()