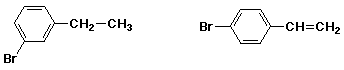
Chem 335 - Winter 2002
Organic Chemistry II
Dr. Carl C. Wamser

1. (15 points) Write complete names for each of the following:
a) 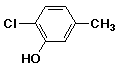
2-chloro-5-methylphenol
b) ![]()
2-(4-bromophenyl)ethanol or
2-(p-bromophenyl)ethanol
Write accurate structures or illustrations for each of the following:
c) p-nitrostyrene
![]()
d) benzyl alcohol
![]()
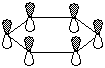
e) the lowest energy pi molecular orbital of benzene
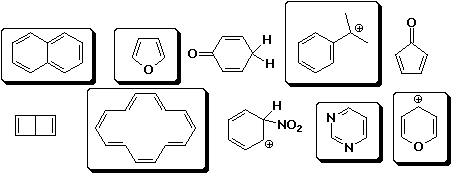
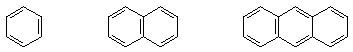
2. (10 points) Circle the compounds below that would qualify as aromatic according to Hückel's rule.
3. (15 points) Arrange the following sets of compounds in
order with respect to the property indicated.
Write "MOST" and "LEAST" under the compounds
with the highest and lowest values of the property.
a) rate of bromination with Br2 + FeBr3
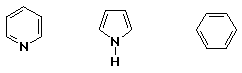
LEAST / / MOST / / MIDDLE
b) SN1 solvolysis in methanol
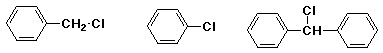
MIDDLE / / LEAST / / MOST
c) SN2 reaction with KCN in DMSO
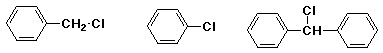
MOST / / LEAST / / MIDDLE
d) rate of nitration
LEAST / / MIDDLE / / MOST
e) rate of Friedel-Crafts acylation
![]()
MIDDLE / / LEAST / / MOST
4. (15 points) Complete each reaction by adding the missing part: either the starting materials, the necessary reagents and conditions, or the final major product.
a) 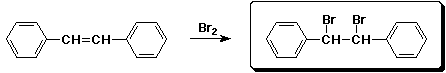
b) 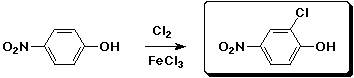
c) 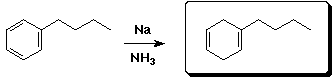
d) 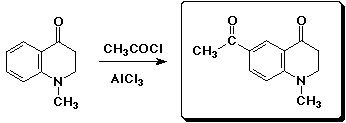
e) 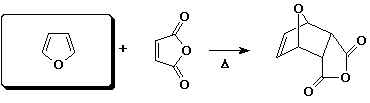
5. (15 points) Write a complete mechanism for the formation of the major product expected from the reaction shown below. Show all steps and all resonance forms for the intermediate involved.
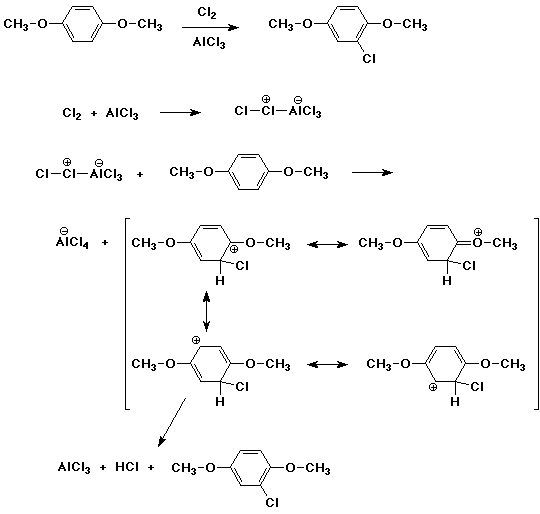
6. (15 points) Amino-substituted benzyl chloride undergoes much faster SN1 reaction than the corresponding unsubstituted benzyl chloride. Explain by showing all the resonance forms for the expected intermediate of the amino derivative.
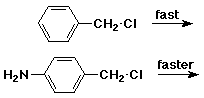
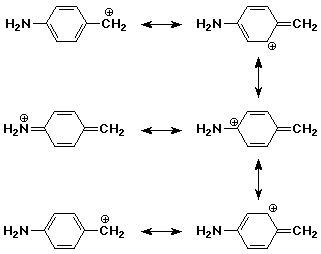
The p-amino substituent provides stabilization to the benzylic cation by resonance. The more stable intermediate is expected to be formed faster (lower Ea).
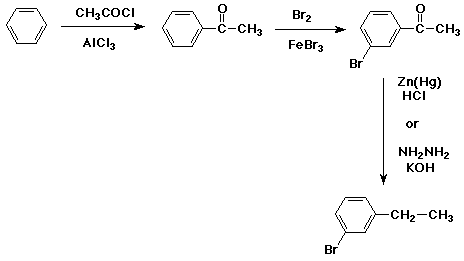
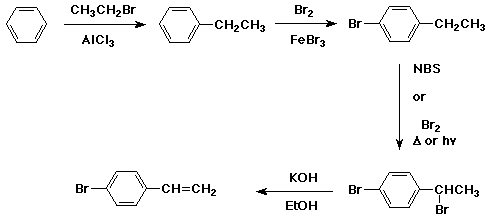
7. (15 points) Write a synthetic sequence of reactions that could be used to make EITHER ONE of the compounds below, starting from benzene. Just do one of the two (or your first effort will be the one graded).