Organic Chemistry II
Dr. Carl C. Wamser
Brown & Foote, pages 495 - 504 :
Problems 13.12 - 13 , 15 - 22 , 27 , 29
1. Predict the general features of the proton and C-13 NMR
spectra of the following compounds. Use the format of question
2 below.
a) diethyl ketone
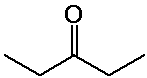
H NMR:
1-2 ppm, area 6, triplet (methyls)
2-3 ppm, area 4, quartet (methylenes)
13C NMR (proton uncoupled):
three peaks: ~30 ppm (methyls), ~50 ppm (methylenes), ~200 ppm
(carbonyl)
b) p-ethylbenzoic acid
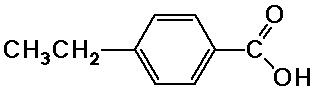
H NMR:
1-2 ppm, area 3, triplet (methyl)
2-3 ppm, area 2, quartet (methylene)
7-8 ppm, area 4, multiplet (aromatic)
11-12 ppm, area 1, singlet (carboxylic acid)
13C NMR (proton uncoupled):
seven peaks: ~30 ppm (methyl), ~50 ppm (methylene), 120-180 ppm
(4 different aromatic carbons), ~200 ppm (carboxylic acid)
c) CH3-O-CH(CH3)-CH2-CH=O
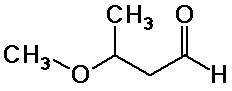
H NMR:
1-2 ppm, area 3, doublet (methyl)
2-3 ppm, area 2, triplet (methylene) (actually a doublet of doublets)
3-4 ppm, area 3, singlet (methoxy)
3-4 ppm, area 1, multiplet (methine - CH) (actually a triplet
of quartets)
9-10 ppm, area 1, triplet (aldehyde)
13C NMR (proton uncoupled):
five peaks: ~30 ppm (methyl), ~50 ppm (methylene), ~70 ppm (methoxy),
~80 ppm (methine - CH), ~200 ppm (carbonyl)
2. Identify the following compounds based on their proton
NMR spectra:
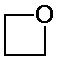
a) C3H6O
2.72 ppm, area 2, quintet
4.73 ppm, area 4, triplet
b) C8H8O2
11.0 ppm, area 1, singlet
7.0 ppm, area 5, multiplet
3.0 ppm, area 2, singlet

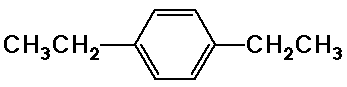
c) C10H14
7.05 ppm, area 4, singlet
2.45 ppm, area 4, quartet
1.05 ppm, area 6, triplet
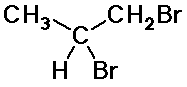
d) C3H6Br2
3.80 ppm, area 1, multiplet
3.50 ppm, area 2, doublet
1.12 ppm, area 3, doublet
e) C6H10
4.82 ppm, area 2, singlet
2.22 ppm, area 4, triplet
1.65 ppm, area 4, triplet