Organic Chemistry II
Dr. Carl C. Wamser
1. (15 points) Write complete IUPAC names for each of the following compounds, including designation of stereochemistry if it is specifically shown:
a) 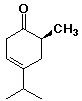
(S)-6-methyl-4-(methylethyl)-3-cyclohexenone
or isopropyl in place of (methylethyl)
b) 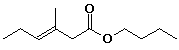
butyl trans-3-methyl-3-hexenoate
or (E) in place of trans
c) 
trans-non-4-en-7-ynal
or (E) in place of trans
2. (15 points) Complete each of the following reactions by adding the missing part: either the starting compound, the necessary reagents and conditions, or the final major product. Show stereochemistry if it is specific.
a) 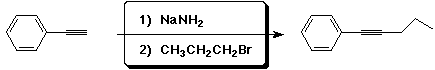
b) 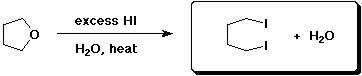
c) 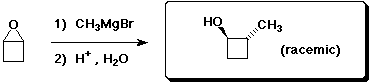
d) 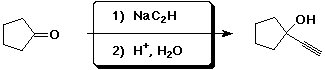
e) 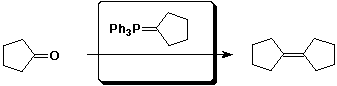
3. (15 points) Complete each of the following reactions by adding the missing part: either the starting compound, the necessary reagents and conditions, or the final major product. Show stereochemistry if it is specific.
a) 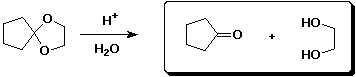
b) 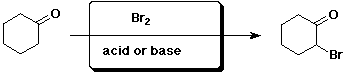
c) 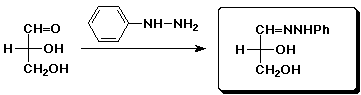
d) 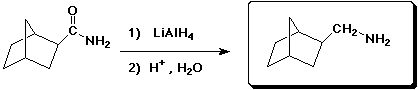
e) 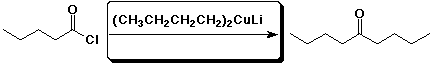
4. (15 points) Complete each of the following reactions by adding the missing part: either the starting compound, the necessary reagents and conditions, or the final major product. Show stereochemistry if it is specific.
a) 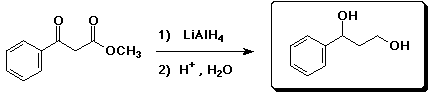
b) 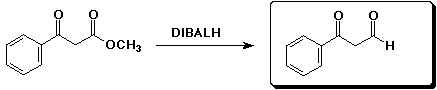
c) 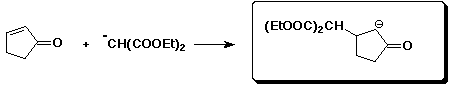
d) 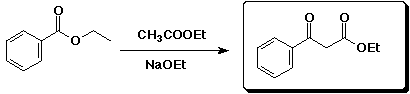
e) 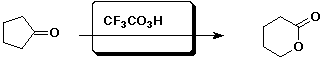
5. (20 points) Write all the resonance forms for the intermediates that would be formed by removing a proton from each of the following:
a) 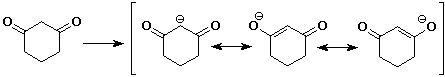
b) 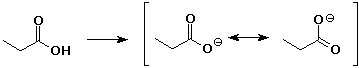
c) 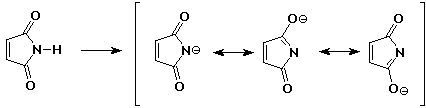
d) 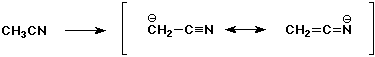
6. (20 points) Write all the resonance forms for the intermediates that would be formed by adding a proton to each of the following:
a) 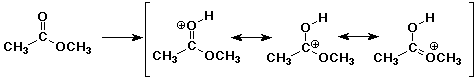
b) 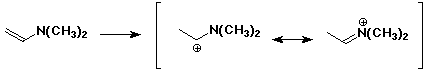
c) 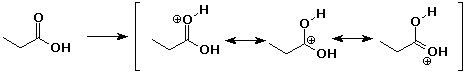
d) 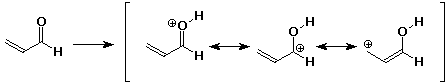
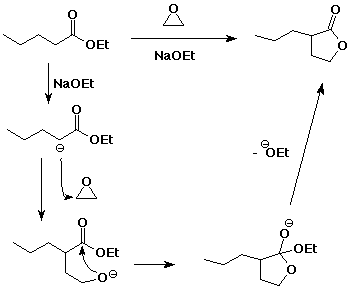
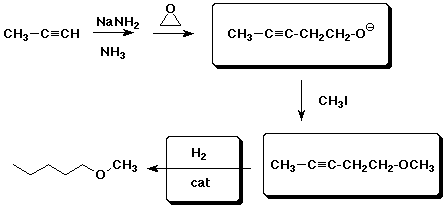
7. (10 points) Complete the reaction pathway shown below:
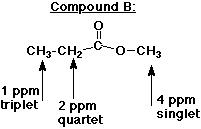
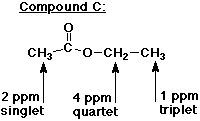
8. (15 points) The three NMR spectra below are of three
different isomers of formula C4H8O2 .
Identify the three isomers and assign each of the NMR absorptions
in each spectrum.

9. (15 points) Based on the previous problem, refer to the expected IR, UV, and mass spectra for compounds A, B, and C.
Mass Spectra:
All three compounds would have this in common:
All have a parent peak at m/z = 88
Suggest how the three might be distinguishable in their mass spectra:
A: M - 29 (loss of HCO)
B: M - 29 (loss of ethyl), M - 31 (loss of methoxy)
C: M - 15 (loss of methyl), M - 45 (loss of ethoxy)
Infrared Spectra:
All three compounds would have this in common:
All have a C=O stretch around 1700-1800 cm-1
Suggest how the three might be distinguishable in their IR spectra:
an aldehyde C=O could be distinct from an ester C=O
A: aldehyde C-H at 2700-2900 cm-1
B: C-O characteristic of CH3-O
C: C-O characteristic of primary C-O
UV Spectra:
All three compounds would likely look very similar in their UV
spectra. What common absorption would be expected?
C=O p -> p* absorption
One of the three isomers of C4H8O2 was NOT 1,4-dioxane (below). Explain what you would expect for its NMR, IR, UV, and mass spectra.
![]()
NMR - singlet at about 4 ppm
IR - only C-H and C-O stretching
UV - no absorption
MS - parent peak at 88
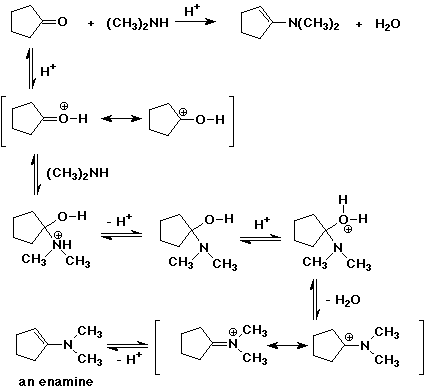
10. (20 points) Write complete mechanisms for the reaction of cyclopentanone with methylamine and with dimethylamine using acid catalysis. The initial stages of your mechanisms should look similar, but different types of products result.

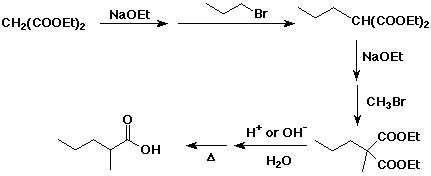
11. (20 points) Show how to prepare the following target compounds from the indicated starting material.

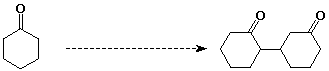
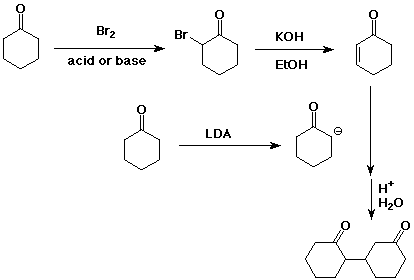
12. (20 points) Write a detailed mechanism that explains how the following reaction takes place.