Organic Chemistry IProfessor
Carl C. Wamser |
|
 |
![]()
Organic Chemistry IProfessor
Carl C. Wamser |
|
 |
![]()
mark every sp with *
put a circle around every sp2
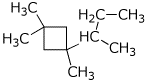
put a square around every sp3C7 H11 Cl O
mark every 1° with *
circle every 2°
square every 3°
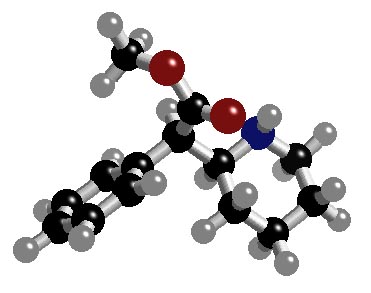
triangle every 4°C11 H21 Br