Organic Chemistry IProfessor
Carl C. Wamser |
|
 |
![]()
Final Exam Answer Key
1. (25 points) Write complete names for each of the following, including absolute stereochemistry.
a) 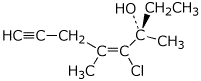
(3S,4E)-4-chloro-3,5-dimethyloct-4-en-7-yn-3-ol
b) ![]()
(1S,3S)-1-methy-3-vinylcyclopentanol
(1S,3S)-3-ethenyl-1-methylcyclopentanol
c) 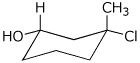
(1S,3R)-3-chloro-3-methylcyclohexanol
d) 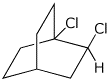
(S)-1,2-dichlorobicyclo[2.2.2]octane
e) 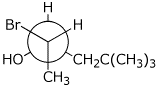
(2S,3R)-2-bromo-5,5-dimethyl-3-hexanol
2. (18 points) Write accurate structures for the following:
a) the conjugate base of 1-butyne
![]()
b) (R)-4-methylspiro[2.5]octane
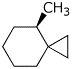
c) a gauche conformation of pentane
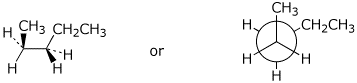
d) a compound with one sp carbon and no triple bonds
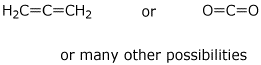
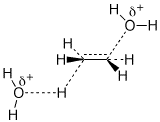
e) the best chair conformation of all-cis-1,2,4-tribromocyclohexane

f) an E2 transition state for dehydration of ethanol
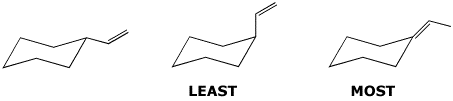
3. (15 points) Arrange each of the following in order with respect to the property indicated. Write “MOST” under the compound with the highest value and “LEAST” under the compound with the lowest value.
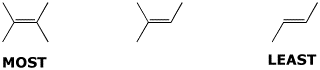
a) stability
b) acidity
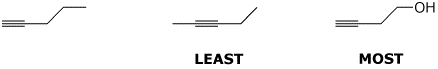
c) nucleophilicity
![]()
d) rate of reaction with iodide ion
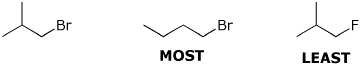
e) rate of reaction with bromine
4. (15 points) Complete each of the following structures so that the structure on the right is the same compound as the structure on the left.
a) 
b) 
c) 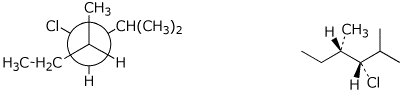
5. (15 points) Complete each of the following reactions by adding the missing part: either the original starting compound, the necessary reagents and conditions, or the final major product. Indicate stereochemistry if it is specific.
a) 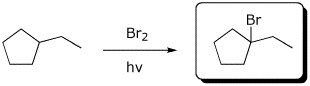
b) 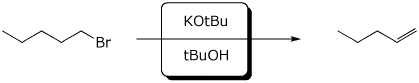
c) 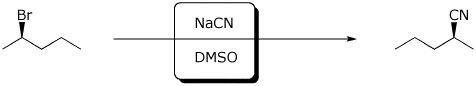
d) 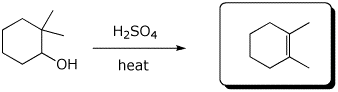
e) 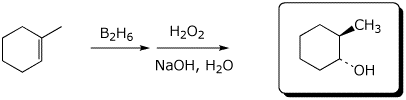
6. (15 points) Complete each of the following reactions by adding the missing part: either the original starting compound, the necessary reagents and conditions, or the final major product. Indicate stereochemistry if it is specific.
a) 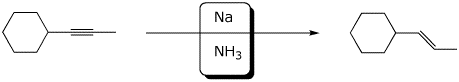
b) 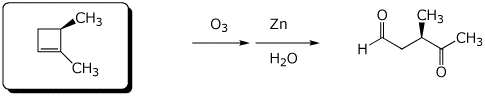
c) 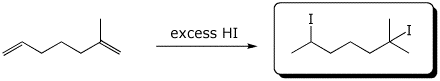
d) 
e) 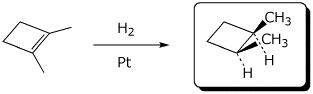
7. (17 points) Complete the following synthetic scheme by adding the missing parts.
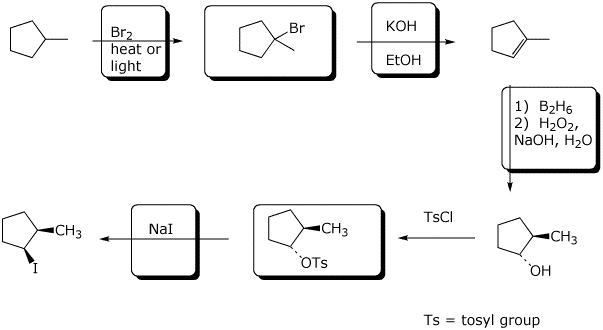
8. (20 points) Show how to synthesize the following target compounds.
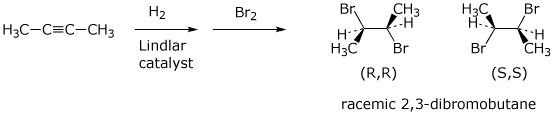
a) cis-3-hexene starting from acetylene as the ONLY source of the carbons in the product

b) trans-2-bromocyclohexanol starting from cyclohexane

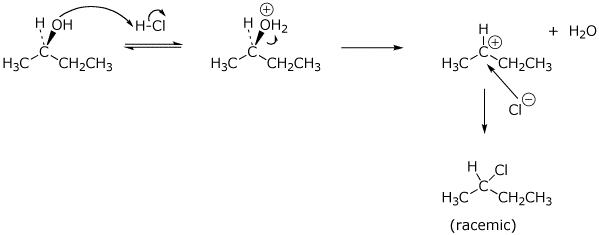
9. (20 points) (R)-2-Butanol could conceivably react in concentrated HCl by any of four possible mechanisms: SN1 , SN2 , E1 , or E2. Write mechanisms to illustrate all of these possibilities, showing each step with electron-pushing arrows and in particular showing the stereochemistry. Just show the mechanisms to give the expected major product.
a) SN1 :
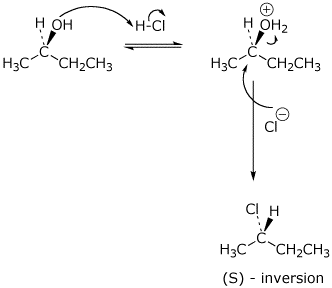
b) SN2 :
c) E1 :
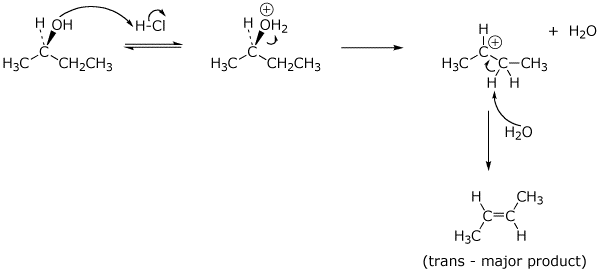
d) E2 :
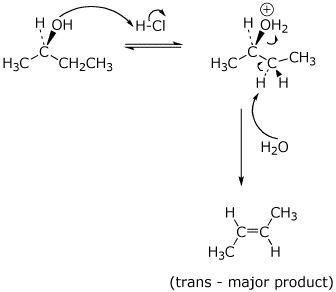
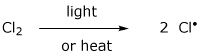
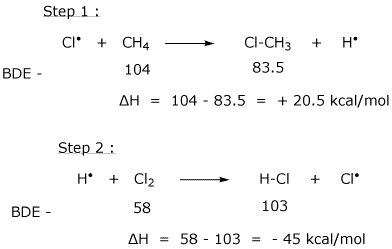
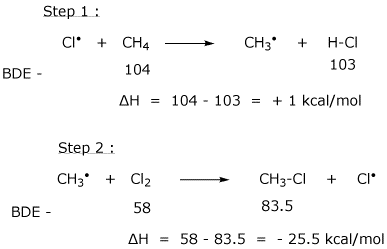
10. (20 points) Consider the following as an alternative mechanism for the chlorination of methane.
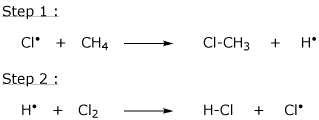
What is the overall reaction accomplished by these two steps?
![]()
Do these two steps constitute a chain reaction?