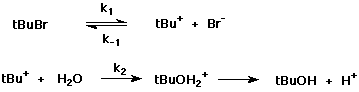Chapter 7 Homework Answers - Haloalkanes. Substitution
& Elimination

Vollhardt & Schore, pp 230 -237
Problems 1 , 3 , 4a , 5 , 6 , 7 , 10 , 11 , 12 , 13 , 14 , 18 , 19 , 22
1. Write a complete mechanism for the conversion of (1R,3S)-1-bromo-1,3-dimethyl-cyclohexane
to a mixture of SN1 and E1 products by heating in methanol solvent.
Show all possible products, including their stereochemistry.
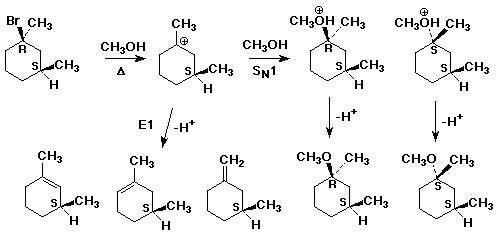
2. The fact that a leaving group can also act as a nucleophile leads
to what is called the "common ion effect". For example, when NaBr
is added to the solvolysis of tert-butyl bromide, the reaction rate is slightly
decreased. Explain this with a complete mechanism. Derive a revised rate
law that explains the rate decrease quantitatively
(see also Problem #20 in the text, page 234, which is similar)
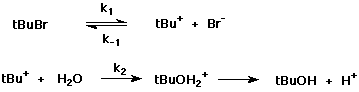
The presence of Br- creates another step ( k-1 ) in which the carbocation
might react. Although this step simply returns the carbocation to starting
material, the rate of product formation will be lower because not every
ionization process will give product. The latter had been true in the simple
SN1 mechanism, where step 1 is the rate-determining step and every cation
is assumed to give product.
without k-1 (normal SN1) :
Rate = k1 [tBuBr]
with k-1 (common ion effect) :
Rate = k1 [tBuBr] x F where F = fraction of carbocation that gives
product
F = k2 [tBu+] [H2O] / ( k2 [tBu+] [H2O] + k-1 [tBu+] [Br-] )
Rate = k1 [tBuBr] k2 [H2O] / ( k2 [H2O] + k-1 [Br-] )
(note that in the absence of Br-, the rate reduces to the normal one)