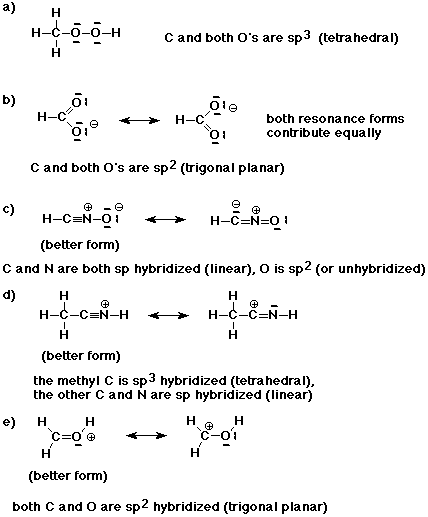Chem 334 - Summer 1998 - Organic Chemistry I
Vollhardt & Schore, pp 38 - 40:
Problems 1 g,h,i ; 2 a-i ; 3 g,h,i ; 6 ; 7f ; 9 ; 10 ; 11 ; 12 ; 17
Write good Lewis structures for the compounds listed below. Indicate the hybridization of each carbon atom and describe the expected geometry. If there are additional resonance forms, show them and indicate whether they contribute equally, or if not, which is the preferred resonance form.
a) CH3OOH
b) HCOO-
c) HCNO
d) CH3CNH+
e) CH2OH+